With the increasing global consumption of plastics, the demand for sustainable recycling technologies has become increasingly urgent - especially for bioplastics such as polylactic acid (PLA). Traditionally, PLA is derived from renewable resources and is expected to become a biodegradable alternative material. However, an efficient recycling method for PLA remains difficult to achieve.
Recent scientific advancements have focused on the photoreforming strategy, which can convert PLA into valuable liquid compounds such as acetic acid, formate, pyruvic acid, and alanine. Despite these advancements, two major obstacles have hindered practical application: the difficulty of efficient depolymerization under mild conditions, and the challenge of extracting high-value products from a mixture containing real impurities.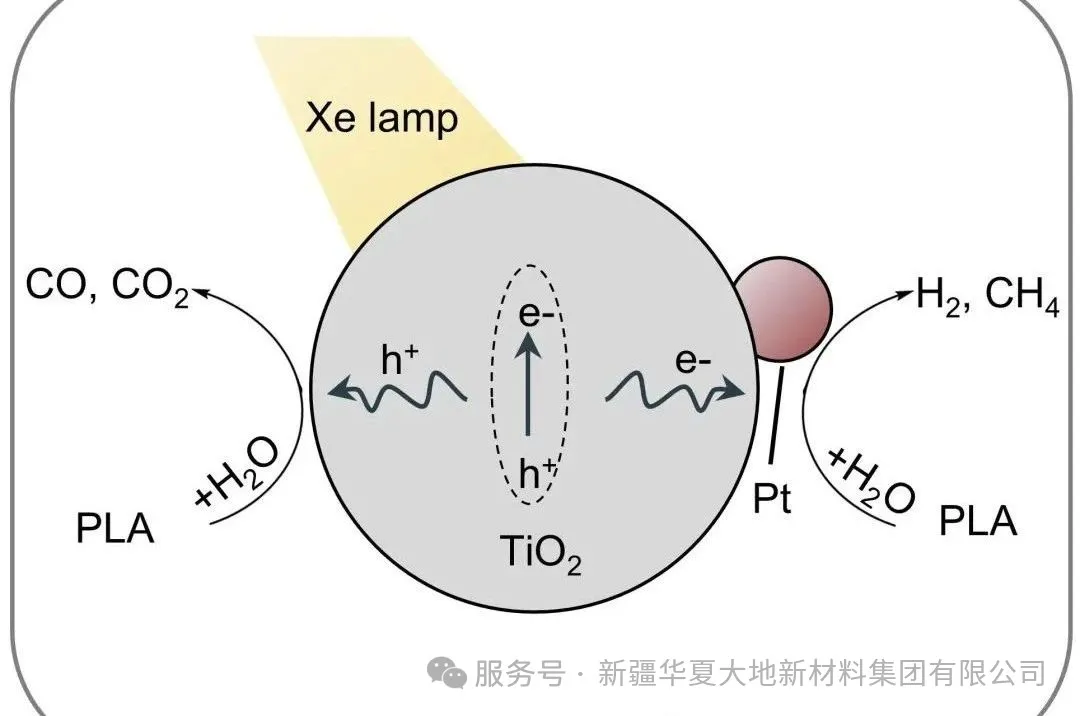
Dual-effect recovery strategy
To address these issues, Professor Zhang Tie-rui from the Institute of Physical and Chemical Technology of the Chinese Academy of Sciences led a research team that introduced an innovative dual-function strategy. Their approach combined photothermal depolymerization with photocatalytic reforming, thus pioneering a more efficient PLA recycling process.
Technical Principle
The core of this breakthrough lies in the collaborative mechanism:
Thermal-hydrothermal effect: During the thermal-hydrothermal heating process, the generated steam helps to depolymerize PLA into lactic acid.
Titanium dioxide (TiO₂) photocatalysis: Under ultraviolet light, the TiO₂ catalyst is activated, converting lactic acid into valuable chemicals such as hydrogen (H₂), carbon monoxide (CO), and methane (CH₄).
This simplified process not only reduces the pollution caused by plastic impurities, but also simplifies the separation of the final product.
The experiment revealed the key products
Through a series of laboratory control experiments, the researchers determined that acetaldehyde and acetic acid were the key reaction intermediates. They discovered:
Hydrogen (H₂) mainly comes from the decarboxylation reaction of lactic acid, as well as the further photoreforming of acetaldehyde/ acetic acid.
Carbon monoxide (CO) and methane (CH₄) mainly originate from the photoreforming of acetaldehyde/acetic acid.
It is worth noting that when ultraviolet light is removed, carbon dots and hydrogen gas are produced - this highlights the crucial role of ultraviolet light in driving the complete reforming process.
The significance of circular economy
This research represents a significant leap forward for bioplastics in their journey towards a circular life cycle. By overcoming the two core challenges in PLA recycling - depolymerization under mild conditions and efficient product separation, this dual-effect strategy simultaneously enhances the scalability and purity of the output.
Furthermore, using readily available catalysts such as TiO₂ and relying on sunlight (through ultraviolet light) provides an environmentally friendly and energy-efficient approach for converting waste PLA into clean fuels and chemical precursors.
Future Direction
As this technology moves from the laboratory to practical application, the key issues include:
Can this method be applied to mixed plastic waste streams?
What are the economic impacts of industrial large-scale production processes?
Can solar energy-enhanced photothermal devices be developed for on-site recovery?
As people's interest in biodegradable plastics and green energy solutions continues to grow, this photothermal-photo catalytic strategy may become the cornerstone of the next generation of plastic recycling innovations.
This research was conducted by the team led by Professor Zhang Tie-rui from the Chinese Academy of Sciences. Their studies have continuously provided inspiration for sustainable new approaches in waste management and chemical production fields.
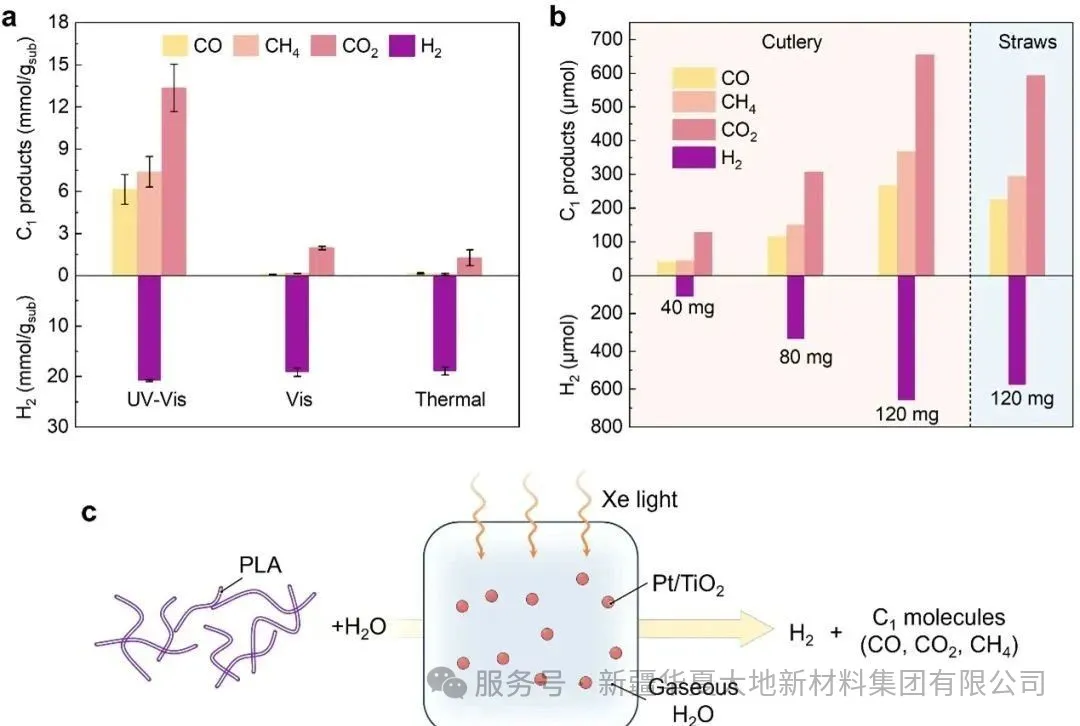 Figure 1. (a) Commercial PLA particles, (b) Real PLA products (tableware and straws) on Pt/TiO₂ for photothermal reforming performance. (c) Schematic diagram of synergistic depolymerization and reforming of PLA on Pt/TiO₂ under xenon lamp irradiation.
Figure 1. (a) Commercial PLA particles, (b) Real PLA products (tableware and straws) on Pt/TiO₂ for photothermal reforming performance. (c) Schematic diagram of synergistic depolymerization and reforming of PLA on Pt/TiO₂ under xenon lamp irradiation.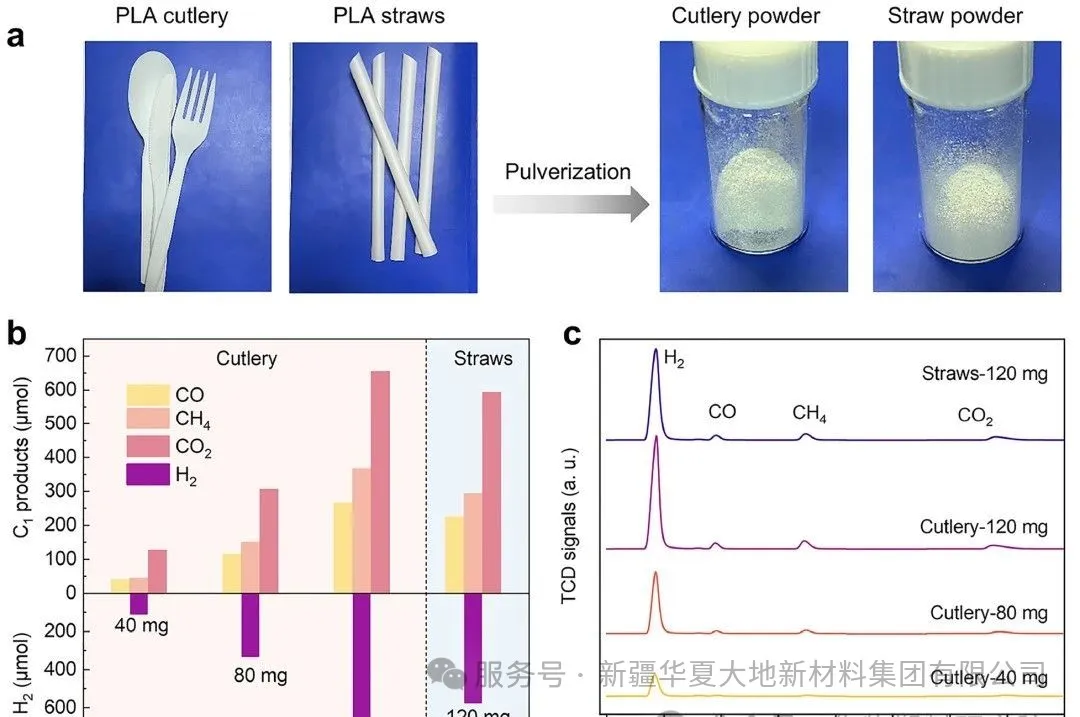 Figure 5 (a) Digital photos of the actual PLA plastic (i.e., PLA tableware and straws) and its corresponding crushed powder. (b) After 4 hours of UV-visible light irradiation at 200°C, the photothermal reforming performance of the actual PLA plastic by the Pt/TiO₂ catalyst. (c) The thermal conductivity detector (TCD) signals corresponding to hydrogen and gaseous C1 products in gas chromatography.
Figure 5 (a) Digital photos of the actual PLA plastic (i.e., PLA tableware and straws) and its corresponding crushed powder. (b) After 4 hours of UV-visible light irradiation at 200°C, the photothermal reforming performance of the actual PLA plastic by the Pt/TiO₂ catalyst. (c) The thermal conductivity detector (TCD) signals corresponding to hydrogen and gaseous C1 products in gas chromatography.