I. Overview of Polylactic Acid
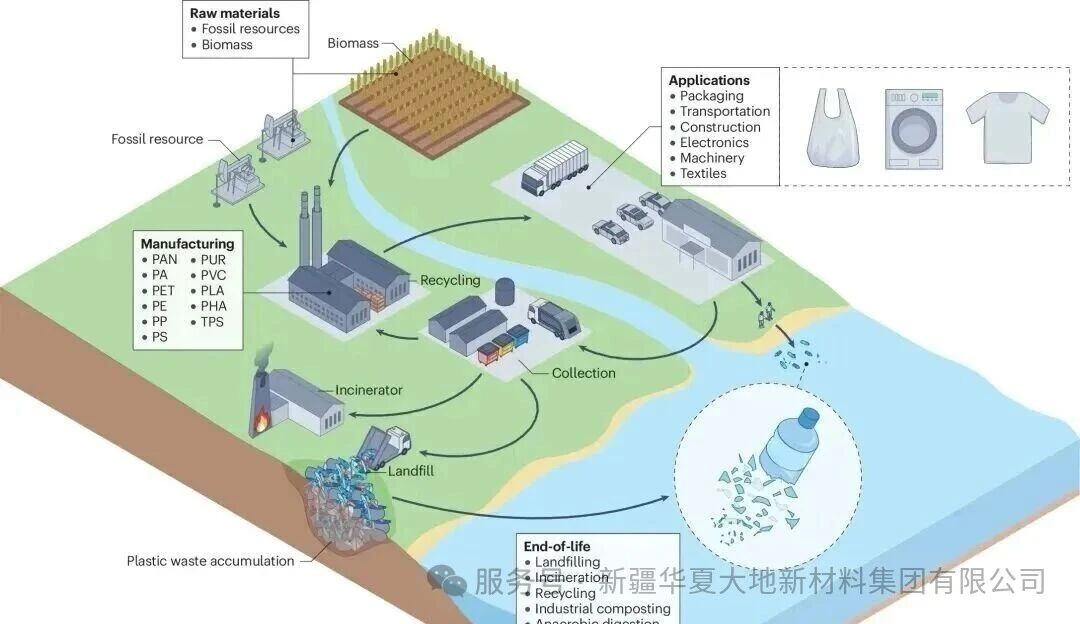
Among various degradable plastics, PLA is regarded as a green material. It is a completely biodegradable and environmentally friendly plastic, which is a polymer obtained through chemical synthesis from lactic acid produced by the biological fermentation of crops (such as corn, starch, wheat straw, etc.). Compared with petroleum-based plastics, the raw materials have a wide range of sources and possess excellent biocompatibility and biodegradability properties. The waste post-poly(lactic acid) plastics can be decomposed into CO2 and H2O by microorganisms in the natural environment under composting conditions. The recycling process is shown in the figure below. PLA has become the most promising biodegradable material at present. The industrial application of PLA can replace petroleum-based plastics, which not only solves the white pollution problem caused by plastics, but also saves the increasingly depleted and non-renewable fossil resources - petroleum.

II. Molecular Structure of Poly(lactic acid)
The various properties of poly(lactic acid) are determined by its own structure. Lactic acid can be classified into L-lactic acid and D-lactic acid based on its optical activity. By mixing equal amounts of the two stereoisomers, racemic lactic acid (D,L-lactic acid) can be prepared. The cyclic dimer formed by dehydration and condensation of the two lactic acids is called lactide. Lactide has four isomers based on different optical activities: L-lactide (L-LA); D-lactide (D-LA); meso-D,L-lactide (meso-LA); racemic D,L-lactide (rac-LA). All of them can form homopolymers. The polymer of D,L-lactide is amorphous and without optical activity; while the polymers of L-lactide and D-lactide have crystallization ability and optical activity.
Industrial-grade PLA is usually synthesized by polymerizing a mixture containing a large amount of L-isomers. This is because most of the bacterial strains used in industrial fermentation mainly produce L-lactic acid. Moreover, due to the difficulty in purifying lactic acid and other issues, PLA molecules usually contain 1-2% D-lactic acid units, but most still exist as PLLA long chains. Structures of lactic acid, lactide and poly(lactic acid) diagrams.

III. Synthesis Methods of Poly(lactic acid)
The commonly used preparation methods for PLA mainly include the following two. According to the different preparation processes, the preparation methods can be classified as direct lactide condensation polymerization method and lactide ring-opening polymerization method. The preparation processes are respectively shown in the figure below.
1. Principle of direct polymerization reaction:

2. Principle of the ring-opening polymerization reaction of caprolactone
In the direct synthesis process of PLA, lactate undergoes dehydration condensation. To obtain high-molecular-weight PLA, a large amount of water needs to be removed from the reaction system. Scientists have discovered a stable Lewis acid catalyst that is resistant to water, with high catalytic efficiency and a product yield of up to 90%. The temperature range for bulk polymerization is 135 ℃ - 175 ℃, and PLA with a molecular weight of 51000 - 71000 g/mol can be obtained; for solution polymerization, a molecular weight of 11000 g/mol can be achieved after a reaction time of 48 hours at 135 ℃.
Researchers used stannous octoate (SnO2ct) as the catalyst for the ring-opening polymerization of D-丙交酯 to prepare PDLA. When the reaction temperature is around 160 ℃, the polymerization time is 20 hours, the catalyst SnO2ct usage is 0.03% of the D-LA usage, and the vacuum degree is 60 Pa, a PDLA with a viscosity average relative molecular mass of 260000 g/mol can be obtained.
VII. Properties and Parameters of Poly(Lactic Acid)