PLA world under the microscope
First of all, directly give the first conclusion, and we imagine that different, also because of the naming rules, polymer materials because of its molecular design can have countless possible structures, different structures present material properties are not the same; From the physical point of view, its crystal hardness, viscoelasticity and other physical properties have very large research space; From the biological point of view, it is also necessary to study the antimicrobial properties of polymer materials and their compatibility with organisms. Therefore, although they have the same polymer name, the clinical manifestations or safety of materials under the same name are worlds apart; It is mainly related to the scientific research level and technological level of raw materials and terminal products; That is, both raw material quality control (polymer chemistry) and preparation process (polymer physics) should be in the lead, and this is not easy!
Polylactic acid, as a representative of polymer materials, is more common in our lives, it contains, such as commonly used environmentally friendly degradable straws, cups, etc., the use of civilian grade raw materials; It also includes highly degradable human implant materials such as bone nails and bone plates, which are made of medical grade raw materials. PLA for injection requires medical-grade raw materials.
Polylactic acid PLA, as a facial filler in the medical beauty industry, has undergone about 20 years of development in 3 eras, which can be generally divided into 1.0, 2.0, 3.0 three different eras; That is, 1.0 crystalline flake; 2.0 Porous microspheres or solid microspheres with generally rough surfaces; 3.0 Solid microspheres with uniform particle size and smooth surface.

Poor quality flour makes it difficult to bake good bread
The representative of 1.0 era - flake crystalline PLA
The representative works of the 1.0 era are, of course, the well-known Derma Veil (not available in China) and the Sculptra (Hong Kong and Taiwan market name: Shu Yan Sui/Su Ran Ya; Mainland region to be listed brand name: Plastic Yancui)
Sculptra, for example, has been approved for 20 years (it was approved by the FDA in 2004 for the "treatment of facial tissue atrophy in AIDS patients"), but in this long timeline, there are always two questions that cannot be avoided:
Can not completely get rid of the difficult to dissolve, easy to produce nodules;
Sculptra, including the whole category of regenerative products, has not occupied a relatively large share in the overseas medical beauty market
Before answering these two questions, we can go back to the raw materials of 1.0 era, that is, where did "bread flour" come from before it was made into "bread"?
Sculptra's raw material supplier is Corbion Purac; As we all know, the raw material supplier is not responsible for the end product and the clinical application of the doctor, which brings two problems:
First, the raw material is subject to Prak, once TA wants to adjust the quality or physical and chemical properties of their products, limited by raw materials, many aspects are hopeless to achieve;
Second, PLac only has three types of PLLA raw materials available, which limits the choice of poly-L-lactic acid raw materials.
Limited by the technical level of Prak raw materials, Gaudemei Sculptra is indeed unable to control the physical and chemical properties of products according to clinical needs.
Therefore, from the "root", the objective reasons of the quality of the raw material itself limit the play of Sculptra in the form of materials, it is difficult to improve, and also explains from the side why after 25 years of Gaudmei, Sculptra's form is "consistent".
Since everyone's "bread powder" comes from one supplier, it has caused a situation in which European and American doctors have to "compromise" and use Sculptra or PLLA injection materials similar to Sculptra, which are difficult to dissolve and have a high risk of nodule, after all, there is really no new material and good material to use.
Let's take a look at the performance of regenerative injection materials in the European and American markets from the perspective of commercial data, and analyze the real reasons.
BCG Report: The European and American medical market is still dominated by hyaluronic acid, and recycled materials are weak year after year.
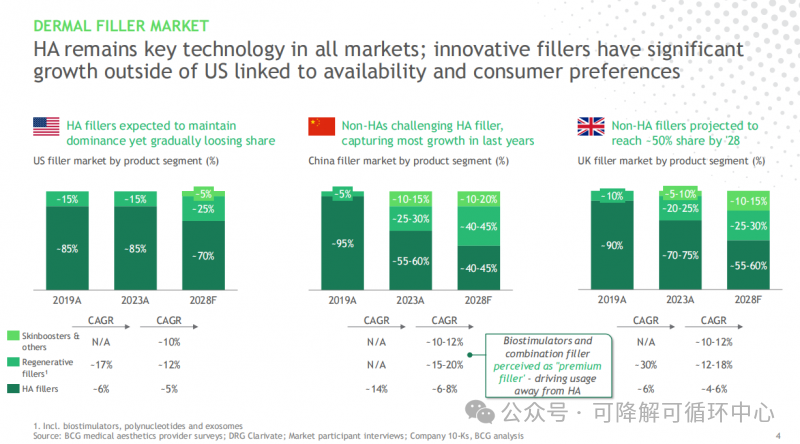 The above is a medical beauty industry market report released by BCG (Boston Consulting Group) in late 2024.
The above is a medical beauty industry market report released by BCG (Boston Consulting Group) in late 2024.
As shown in the figure: in the US market, until 2023, traditional hyaluronic acid fillers have always occupied the dominant position of injection materials, and this situation is expected to change significantly after 4 years; In the United Kingdom, the representative country of the European medical beauty market, for example, in 2023, traditional hyaluronic acid still occupies more than 70% of the market share, and this dominant position will probably be shaken in 28 years. The pattern of injection materials in China's medical beauty market is quite different. 2021, as the first year of regeneration, has successively launched the Iveran Children's beauty needle, Yiyanshi girl's needle and Luteo. Due to the progress of material form brought by the advancement of technology, the Chinese medical beauty market has gained new vitality under the guidance of new regenerative materials in the 2.0 and 3.0 era.
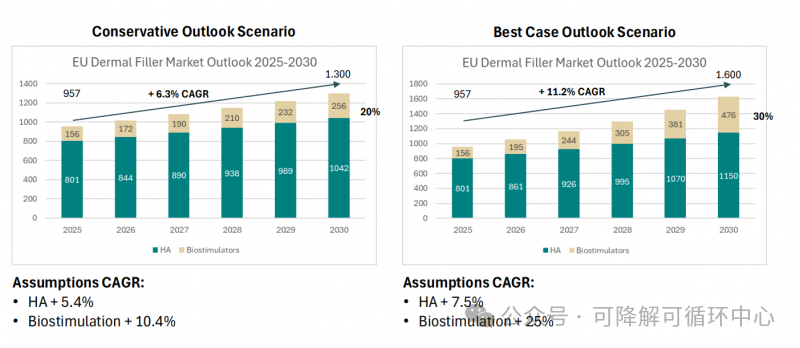 As mentioned above, another data survey of the global medical beauty market published in 24 years shows that the entire European filler market, even if it is released to make forecasts, by 2030, although hyaluronic acid will rise slowly, it will still occupy 70% of the market share.
As mentioned above, another data survey of the global medical beauty market published in 24 years shows that the entire European filler market, even if it is released to make forecasts, by 2030, although hyaluronic acid will rise slowly, it will still occupy 70% of the market share.
Thus, the general trends and status quo indicate that: In today's European and American medical markets, it is because of the safety issues of Sculptra led regenerative materials and the weak product power that hinder the overall commercial performance of regenerative materials in overseas markets, and it is impossible to reach the situation that the Chinese market leads the rapid development of regenerative market due to product advantages. Doctors have to choose traditional hyaluronic acid as the mainstream injection materials. Traditional hyaluronic acid is still the largest category that dominates the market.
The representative of 2.0 era - microsphere PLA
With the progress of The Times, people have found that the shape of the material determines the clinical performance of the material. The crystalline PLA in the European and American markets has been criticized, so in the Asia-Pacific region, some upstream material suppliers have made improvements on the basis of 1.0, and then PLA has evolved from flake crystals into spheres. Among them, the representative of the 2.0 era is PDLLA microsphere (also includes some raw material control and preparation process has not been refined in place of PLLA microsphere products), the PDLLA microsphere in the 2.0 era shows a kind of porous microsphere with a rough surface under the microscope.
What's good about 2.0?
First of all, the relevant literature shows that the sphere of the 2.0 era does make the doctor's redissolution easier, which also means that the probability of nodules is lower than the 1.0 era products represented by Sculptra.
But what's wrong with 2.0? It can be said from two dimensions:
Material composition
Why not just make a PLLA microsphere, but introduce PDLA D-lactic acid, which is not so good, and evolve into PDLLA microspheres? It is well known that the human body does not have the enzyme to metabolize poly-d-lactic acid (PDLA), and the D-lactic acid degraded by poly-D-D-lactic acid (PDLA) can accumulate and cause acidosis, resulting in neurotoxic effects. Therefore, D-lactic acid is prohibited in food for infants and young children, and the daily intake of D-lactic acid for adults is limited to less than 100mg/kg body weight.
For the medical beauty industry, the customer group itself is a healthy population, and the suppliers of upstream medical beauty materials should follow the "non-relief principle" to develop extremely safe products that will never harm the health of the beauty seekers under the premise of doctor's standardized operation.
If it is not made pure, there is a possibility that the optical rotation purity of the production process can not be controlled, that is to say, due to the limitations of the production process, there is no way to make a pure left-handed microsphere in the 2.0 era, and another possibility is that the hardness of the porous sphere itself has a problem, because to solve the fragility of the structure of the sphere itself, Therefore, we have to add PDLA on the basis of PLLA to add its own hardness.
Material form
Secondly, because its structure is a porous microsphere with a rough surface, its degradation process may be uncontrollable, resulting in two undesirable situations:
In the process of degradation, the sphere is impacted by external forces and suddenly collapses. After collapse, flake crystals similar to those in the 1.0 era may be formed again, resulting in nodules and other adverse reactions.
Because it is a "rough surface" porous microsphere, it generates static electricity after friction, and it is easy to agglomerate, and it will still be further wrapped by macrophages after agglomeration, forming inflammatory tissue, or causing nodules.
The above is just an inference made from the shape of the material, what is the difference between PLLA, PDLA and PDLLA from the perspective of "inflammation"?
Let's take a look at animal experiments.
In the following study, researchers prepared three microspheres (MSs) consisting of PDLA, PLLA, and PDLLA with particle sizes of 5μm, 10μm, and 20μm, respectively, to demonstrate their different biological functions as dermal filling materials. After subcutaneous injection in guinea pigs, PLLA-MS was found to induce the least inflammatory response, and its induced levels of the pro-inflammatory cytokine IL-1β were only 0.3 or 0.7 times those induced by PDLA or PDLLA-MS, respectively. More importantly, the effect of PLLA-MS on collagen regeneration was more significant, 1.4 times or 1.1 times that of PDLA-MS or PDLLA-MS, respectively. The size of PLA-MS did not affect the levels of inflammation and collagen regeneration. Therefore, PDLLA is difficult to get rid of the dilemma of inflammatory regeneration, whether from morphological inference or animal tissue sections in the literature.
The results confirm that if nodules and other adverse reactions caused by morphological problems of 1.0 PLLA materials represented by Sculptra are solved, pure PLLA microspheres will show excellent superiority and safety as collagen stimulators.
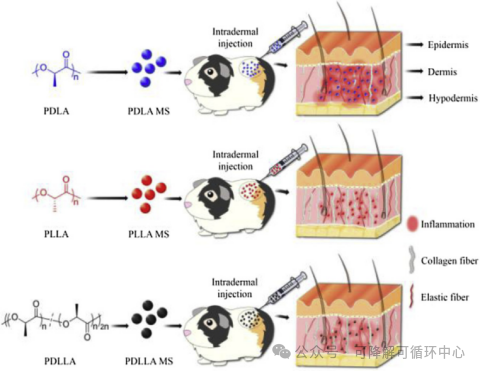
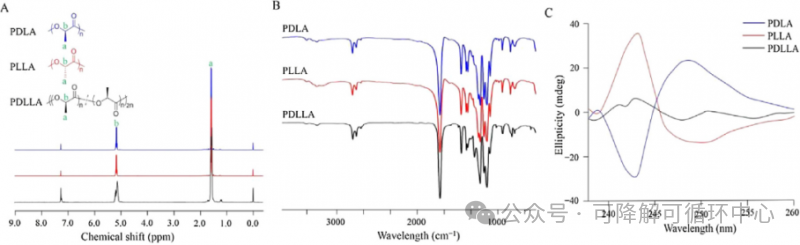 The representative of the 3.0 era - pure PLLA smooth solid microspheres
The representative of the 3.0 era - pure PLLA smooth solid microspheres
Professionals said that the preparation and mass production of medical polylactic acid raw materials has high technological barriers. Taking the preparation of L-polylactic acid as an example, the material is very sensitive to water, unstable, easy to decompose, and difficult to mass produce with high purity and high yield. Therefore, in the selection of medical polylactic acid raw materials, the focus needs to pay attention to the purity, monomer (lactic acid) residue, solvent residue and other parameters, to meet these high standards is not easy. Therefore, it is not easy to get a perfect PLA product.
So, what does a pure PLLA microsphere in the 3.0 era look like?
 In form, it is a solid microsphere with a smooth surface, which not only does not need to be redissolved for a long time like the traditional PLLA in the 1.0 and 2.0 era, leading to increased risks such as infection (long resolution time also means that the product is poor in solubility, and it is more likely to produce nodules after application), but also avoids the formation of "nasty foam". These "nasty bubbles" can contain clumps of particles, which can not only clog the needle, but also increase the risk of nodules.
In form, it is a solid microsphere with a smooth surface, which not only does not need to be redissolved for a long time like the traditional PLLA in the 1.0 and 2.0 era, leading to increased risks such as infection (long resolution time also means that the product is poor in solubility, and it is more likely to produce nodules after application), but also avoids the formation of "nasty foam". These "nasty bubbles" can contain clumps of particles, which can not only clog the needle, but also increase the risk of nodules.
 Description: The above redissolution photos are redissolved in accordance with the recommended concentration of the product manual.
Description: The above redissolution photos are redissolved in accordance with the recommended concentration of the product manual.
Very obvious flocculent substances remain on the bottle wall after the redissolution of products in the 1.0 era; spheres are difficult to dissolve after the redissolution of products in the 2.0 era, and the liquid is very viscous after the redissolution; the bottle wall of products in the 3.0 era is clean after the redissolution, and the injected liquid shows good dispersion and no precipitation after the redissolution.
In the 3.0 era, a very important part of the process is to control the rate of microsphere degradation. The preparation process of microspheres in the 3.0 era controlled the uniform degradation of microspheres within 2 years (106 weeks). That is to say, in these two years, the degradation rate of the microspheres remains uniform, so this means that the clinical effect is stable: there will not be a particularly long effect for some people, and a particularly short effect for some people. This is somewhat like a "slow release" effect, that is, continuous release of lactic acid, which exerts a stable regeneration effect, achieving self-stable collagen growth and lower inflammatory response, achieving a gradual, natural effect.
Here is a popular science: the circular smooth sphere has good histocompatibility and causes a low immune response. Smooth spheres are less prone to agglomeration.
In addition, it is precisely because of the superior shape of the solid sphere of PLLA in the 3.0 era that doctors have improved its redissolution and injection methods in clinical practice, which has improved the comfort of injection for beauty seeking and promoted the recovery of tissue function. Compared with 1.0 PLLA, there are obvious differences in safety and effect, which has changed the previous mediocre market performance of polylactic acid. It has really set off a new wave of the regeneration era in China, and has therefore been recognized by doctors and beauty seekers.
In SUMMARY:
PLA as a global popular recycled material, from 1.0, 2.0 to 3.0, we have seen the material progress brought by the improvement of the preparation process and the improvement of safety. We also found that only at the same time in raw materials and end products, the iteration of the process can promote the improvement of the product, from the raw materials to the end products, from the source of collaborative quality control, in order to produce good products from the "root".
With the development of science and technology, the advancement of marketization and information technology in China, and the significant improvement of consumer cognition, as an upstream manufacturer, it is necessary to continue to take product power as the core, take consumer interests and needs as the guidance, and insist on continuous improvement in research and development and product upgrading.
More than a decade ago, the era of hitting the Chinese market in exchange for high profits only with the concept of marketing gimmicks, or obsolete products that are not sustainable overseas, is gone forever.








