On May 7, 2025, the Department of Food Safety Standards and Monitoring and Evaluation issued the "Announcement on 11 'Three New Foods' Including Sakura Polyphenols" (No. 3 of 2025), introducing three new food-related product varieties including docodiamide.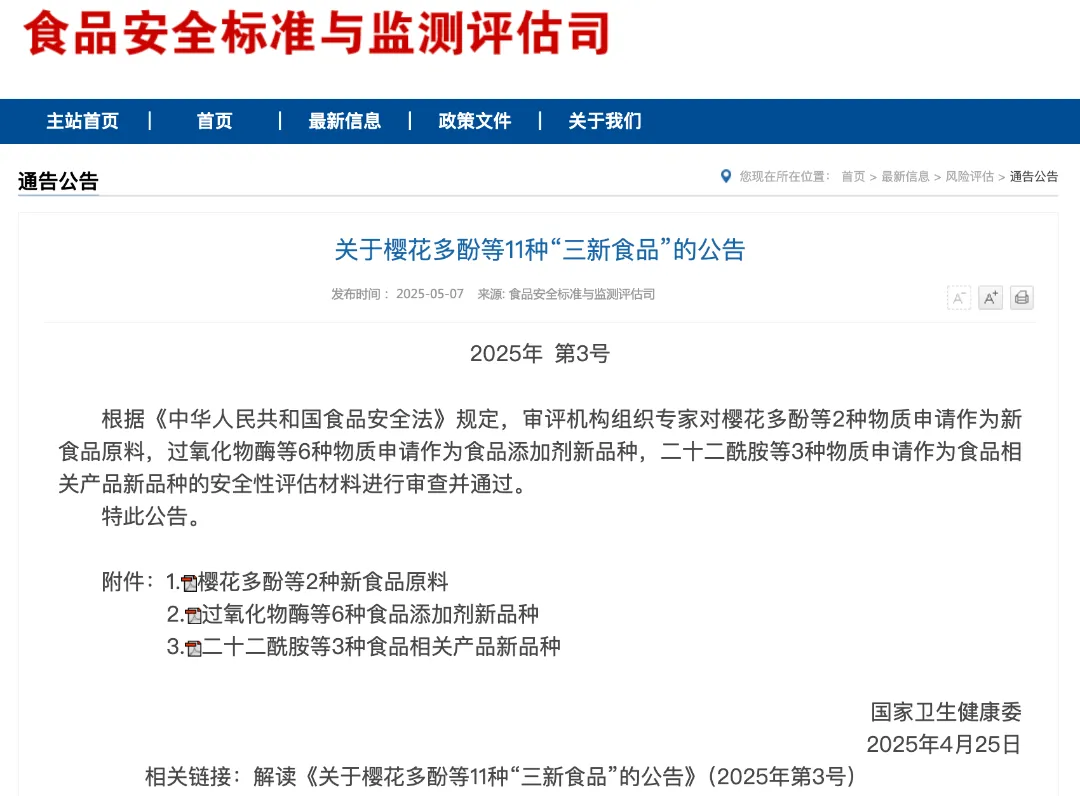
The official interpretation information released is as follows:
1. Expand the application scope of additives for food contact materials and products
● Docodiamide
The "National Food Safety Standard - Standard for the Use of Additives in Food Contact Materials and Articles" (GB 9685-2016) has approved that docodiamide can be used in various plastic materials and articles such as polyethylene (PE), polypropylene (PP), polystyrene (PS), and polyvinyl chloride (PVC).
This announcement expands the application scope of docotanamide to include polybutylene terephthalate (PBT) and polylactic acid (PLA) plastic materials and products for food contact. Docodiamide is used as a lubricant and anti-sticking agent in PBT and PLA plastic materials and products, which can reduce the friction or adhesion force on the polymer surface and play a role in smoothness and anti-sticking. After safety assessment, PBT and PLA single-use plastic materials and products with docodiamide added can be safely used by all people when filled at room temperature (including hot filling, pasteurization or other heat treatment) and stored at room temperature for a long time. Other usage requirements shall be implemented in accordance with the provisions of the announcement.
The U.S. Food and Drug Administration, the European Commission and Mercosur all permit the use of this substance in PBT and PLA plastic materials and products for food contact.
2. New varieties of resins for food contact materials and products
●Polymer of 1, 4-benzoic acid with 2-methyl-1, 3-propylene glycol and 4, 8-tricyclic [5.2.1.02,7] decanedimethanol
This announcement approves the polymer of 1, 4-phthalic acid with 2-methyl-1, 3-propylene glycol and 4, 8-tricyclic [5.2.1.02,7] decanedimethanol as a new variety of resin for food contact for use in coatings and coatings. The coating produced with this substance as raw material has good corrosion resistance.
After safety assessment, the coating produced with this substance as raw material can be safely used at a temperature not exceeding 131℃. In view of the insufficient safety data of polymers of 1, 4-phthalic acid with 2-methyl-1, 3-propylene glycol and 4, 8-tricyclic [5.2.1.02,7] decanedimethanol for the infant population, considering the principle of risk prevention, the use of such materials in the production of food contact materials and products specifically for infants and young children is restricted. Other usage requirements shall be implemented in accordance with the provisions of the announcement.
Both the European Commission and Mercosur permit the use of this substance in food contact paints and coatings.
Polymers of 1, 3-phthalic acid with 1, 4-phthalic acid, phthalic anhydride, maleic anhydride, 1, 2-propanediol, 1, 2-ethylene glycol and diethylene glycol, and copolymers of styrene
This announcement approves the copolymer of 1, 3-phthalic acid with 1, 4-phthalic acid, phthalic anhydride, maleic anhydride, 1, 2-propanediol, 1, 2-ethylene glycol and diethylene glycol with styrene as a new type of food contact resin for use in unsaturated polyester resin (UP) plastic materials and products.
The UP plastic materials and products produced from this substance have good mechanical properties and acid resistance. After safety assessment, UP plastic materials and products produced from this substance can be safely used under conditions where the temperature does not exceed 70℃, the ethanol content of the food they come into contact with does not exceed 20%, and the S/V in contact with food does not exceed 2dm²/kg. Given the insufficient safety data on the use of copolymers of 1, 3-phthalic acid, 1, 4-phthalic acid, phthalic anhydride, maleic anhydride, 1, 2-propanediol, 1, 2-ethylene glycol and diethylene glycol with styrene in the infant population, considering the principle of risk prevention, the use of such materials in the production of food contact materials and products specifically for infants and young children is restricted. Other usage requirements shall be implemented in accordance with the provisions of the announcement.
The U.S. Food and Drug Administration, the European Commission, the Ministry of Health, Labour and Welfare of Japan, and the Swiss Federal Agency for Food Safety and Veterinary Services all permit the use of this substance in unsaturated polyester resin (UP) plastic materials and products for food contact.