Waste plastic pollution has become a global environmental crisis that urgently needs to be addressed. As a key basic material in modern industry, plastic has fully permeated core industries such as food packaging, automobile manufacturing, and electronic appliances. Data shows that due to the combined driving forces of population expansion, consumption upgrade, and industrial technological innovation, the global annual plastic production has increased from less than 300 million tons to 540 million tons in the past decade, and it will continue to maintain a high growth trend in the future.At present, the disposal of waste plastics has become a major challenge restricting sustainable development. The existing waste plastic disposal system mainly includes four technical paths: physical recycling (blending and modifying old and new materials to produce daily necessities and building materials), chemical recycling (catalytic cracking to prepare monomer raw materials or high-value materials), energy recovery through incineration, and centralized landfill. However, from the perspective of economic and environmental protection, physical recycling has the defect of performance degradation, and incineration and landfill directly violate the carbon neutrality goal. Although chemical recycling temporarily faces a few technical bottlenecks (such as high cost and the poisoning effect of impurities on precious metal catalysts), the development of new environmentally friendly catalytic systems and continuous technological improvements and iterations can solve these problems and promote the construction and sustainable development of a closed-loop resource regeneration system.
01 PET catalytic alcoholysis
Recently, the team of Mei Qingqing from Zhejiang University has developed an efficient and low-carbon KHCO₃/guaiacol co-catalytic system, which can achieve efficient PET methanolysis under mild conditions of 120°C and 0.6 MPa. The yields of DMT and ethylene glycol are as high as 94% and 98% respectively. The research through systematic characterization and theoretical calculations reveals that the phenolic hydroxyl groups can stabilize the key intermediates through hydrogen bonding, reduce the reaction energy barrier, and effectively inhibit the reverse reaction. It clarifies the key catalytic assistance role of guaiacol in improving the reaction selectivity and efficiency. This system shows wide substrate adaptability, especially suitable for the selective depolymerization of blended textiles, and has good scalability and application prospects. A green co-catalytic system centered on KHCO₃/guaiacol was constructed, breaking through the technical barriers of high temperature and high pressure, strong corrosion, etc. faced by traditional PET methanolysis. At the mechanism level, unlike traditional acid catalysis or solvent-assisted pathways, through systematic characterization and DFT calculations, we have for the first time revealed the new mechanism of guaiacol as a co-catalyst, which stabilizes the reaction intermediates through hydrogen bonding and inhibits the reverse reaction. This provides a new idea for the design of efficient and controllable catalytic systems.
02 Photocatalytic of polylactic acid
Polylactic acid (PLA), as a mainstream biodegradable plastic, is widely used in packaging, textiles and other fields. However, although PLA is biodegradable, its degradation rate in the natural environment, especially in the marine environment, is extremely slow. This results in a large amount of PLA waste plastic being discharged into the environment, becoming a persistent pollution source. Through photocatalysis, the waste plastic can be converted into high-value chemicals and energy, providing an environmentally friendly and sustainable plastic recycling approach. Atomic dispersion catalysts, as an emerging catalytic material, have become a research hotspot for improving photocatalytic efficiency due to their 100% atomic utilization, tunable electronic structure and quantum size effect.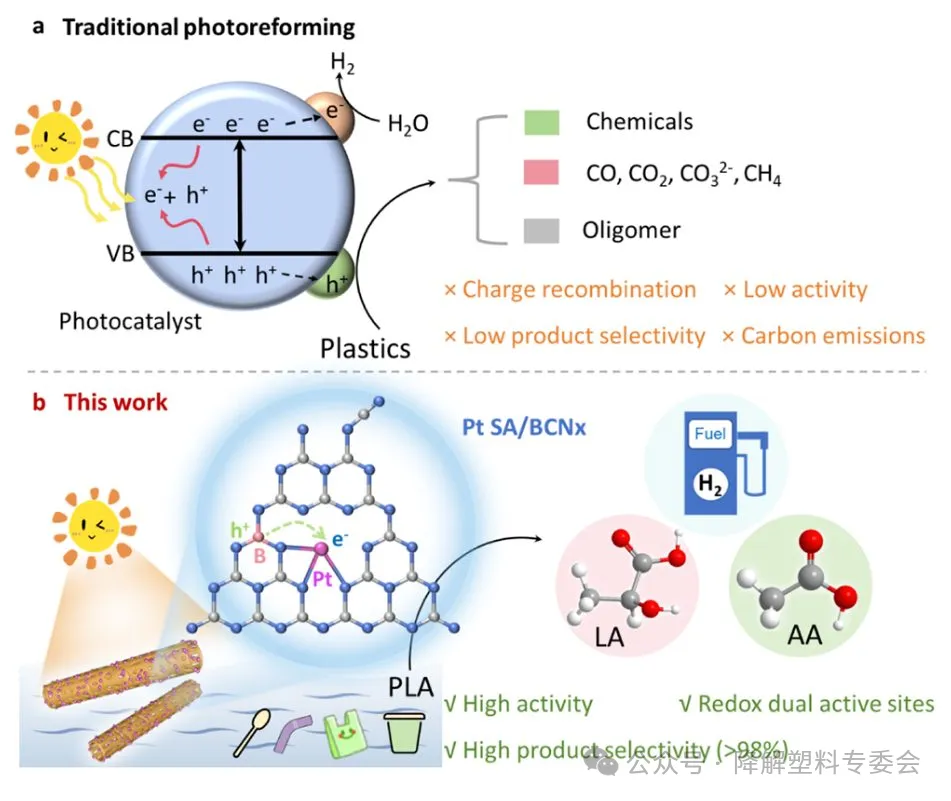
The research team from Tianjin University and Nankai University proposed a Pt-B dual-site atom-dispersed catalyst for efficient photoreforming of PLA waste plastics in seawater. The study demonstrated that the synergistic effect between the Pt and B dual sites significantly enhanced the catalytic performance. Specifically, the enriched photogenerated holes at the B site could effectively activate the C-H and C-C bonds in PLA, selectively degrading PLA to produce acetic acid (AA); while the Pt site promoted the generation of hydrogen (H2). Thus, an efficient hydrogen evolution rate (993 μmol g⁻¹·h⁻¹) and acetic acid yield (300 μmol g⁻¹·h⁻¹) were achieved, with an acetic acid selectivity of over 98%. Moreover, this catalytic system not only performed well in the large-scale treatment of PLA plastics but also successfully treated PLA microplastics in natural seawater, indicating its potential application in real environments. This new method combines the green hydrogen industry with plastic upgrading, providing a promising solution for the restoration of marine environments and the recycling of dispersed plastics. Future work should focus on the design of photocatalytic devices to enhance their potential for efficient and large-scale application in practical scenarios.
03 Electrocatalytic plastic recycling and reuse
Recently, the research team led by Professor Shao Mingfei from Beijing University of Chemical Technology has made a significant breakthrough in the field of electro-catalytic plastic recycling and resource utilization. They have for the first time achieved a full-process electro-catalytic conversion of polyethylene terephthalate (PET) waste materials into biodegradable polymer polyglycolic acid (PGA), and have achieved stable operation for 1000 hours at an ampere-level current density, while simultaneously producing hydrogen. The research team first constructed an AuPd alloy electro-catalyst with synergistic active sites. Here, Au acts as the catalytic site to promote the generation of active oxygen (OH*), and Pd acts as the adsorption site to enhance the interface adsorption and enrichment of EG. Under this synergistic effect, efficient EG electro-oxidation was achieved at an ampere-level current density (>1 A/cm2), with a GA selectivity of up to 94%, and stable operation for 1000 hours was achieved. The performance is significantly superior to the reported catalytic systems.







