You may not have heard of "polylactic acid fiber", but you must have seen its applications: the skin-friendly fabric in baby and maternity clothing, the absorbable material for surgical sutures, and the degradable packaging for takeout boxes... This green fiber called "lactic silk" is quietly changing our lives.
It comes from biomass raw materials such as corn and straw, and can eventually degrade into water and carbon dioxide, causing almost no pollution throughout the process. But do you know? From a pile of crops to a single fiber, there are many technological secrets behind it. Today, let's delve into the "birth code" of polylactic acid fibers and see how this "disappearing fiber" is made.
 The Molecular Structure Holds Secrets: Why Only PLLA Can Be Spun into Practical Fibers?
To discuss the technology of polylactic acid fibers, we must first start with their "genes" — the molecular structure.
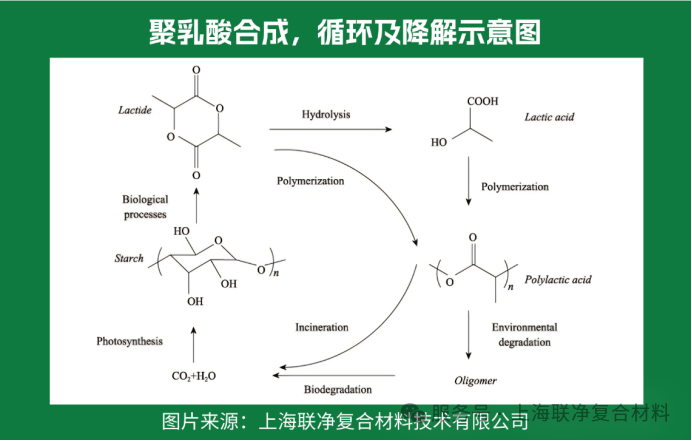
The macromolecules of polylactic acid (PLA) have a linear straight-chain structure without complex side groups, just like a smooth rope. What makes it special, though, is that the structural units contain "chiral carbon atoms" — similar to human left and right hands, which are symmetrical yet cannot be perfectly overlapped. This gives polylactic acid macromolecules three "personalities": poly-D-lactic acid (PDLA), poly-L-lactic acid (PLLA), and poly-DL-lactic acid (PDLLA).
The Molecular Structure Holds Secrets: Why Only PLLA Can Be Spun into Practical Fibers?
To discuss the technology of polylactic acid fibers, we must first start with their "genes" — the molecular structure.
The macromolecules of polylactic acid (PLA) have a linear straight-chain structure without complex side groups, just like a smooth rope. What makes it special, though, is that the structural units contain "chiral carbon atoms" — similar to human left and right hands, which are symmetrical yet cannot be perfectly overlapped. This gives polylactic acid macromolecules three "personalities": poly-D-lactic acid (PDLA), poly-L-lactic acid (PLLA), and poly-DL-lactic acid (PDLLA).
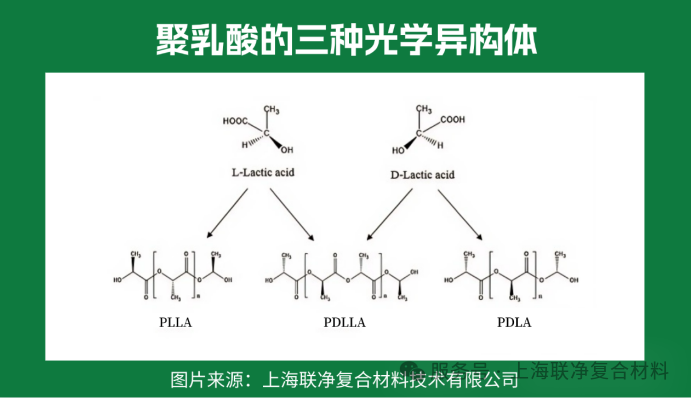 Why is PLLA the sole choice for industrial fiber production? The key lies in "crystallinity". PLLA features "isotactic configuration" — its molecular chains are arranged like neatly aligned soldiers, enabling tight packing to form crystalline regions, much like neatly folded quilts, which grants it stable structure and sufficient strength. In contrast, PDLA has poor crystallinity, and PDLLA is even more disorderly like "scattered soldiers", completely failing to support the mechanical properties required for fibers.
To put it metaphorically: the molecular chains of PLLA are a "well-trained team" that can cluster together to form strong fibers; the other two are like "loose sand" that breaks when pulled during spinning. This is why over 90% of polylactic acid fibers worldwide use PLLA as the raw material.
From Resin to Fiber: Why Has Melt Spinning Become the "Optimal Solution" for PLA Industrialization?
With the appropriate raw material PLLA, the next step is to transform solid polylactic acid resin into slender fibers. Bio-based polylactic acid (PLA) fibers are synthetic fibers made from polylactic acid (PLA) through spinning processes (melt spinning, wet spinning, dry spinning, dry-wet spinning, electrospinning, etc.). Currently, there are two mainstream spinning methods: melt spinning and solution spinning, with melt spinning being the most dominant process.
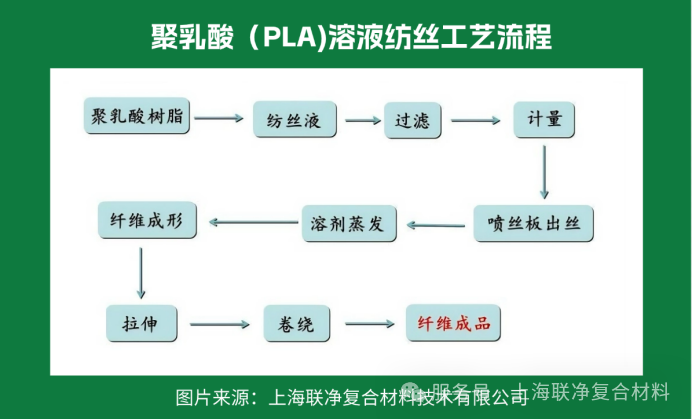
Solution spinning is like "making coffee": the polylactic acid resin is dissolved in a special solvent to form a colloidal solution, which is then extruded from the spinneret and solidified into fibers in the solvent. Fibers produced by this method have better mechanical properties, similar to a well-kneaded dough with excellent toughness. However, the problem is that solvent recovery is difficult and costly. Moreover, solvent volatilization in the spinning workshop requires workers to wear protective clothing, making it suboptimal in terms of both environmental protection and economic efficiency.
Why is PLLA the sole choice for industrial fiber production? The key lies in "crystallinity". PLLA features "isotactic configuration" — its molecular chains are arranged like neatly aligned soldiers, enabling tight packing to form crystalline regions, much like neatly folded quilts, which grants it stable structure and sufficient strength. In contrast, PDLA has poor crystallinity, and PDLLA is even more disorderly like "scattered soldiers", completely failing to support the mechanical properties required for fibers.
To put it metaphorically: the molecular chains of PLLA are a "well-trained team" that can cluster together to form strong fibers; the other two are like "loose sand" that breaks when pulled during spinning. This is why over 90% of polylactic acid fibers worldwide use PLLA as the raw material.
From Resin to Fiber: Why Has Melt Spinning Become the "Optimal Solution" for PLA Industrialization?
With the appropriate raw material PLLA, the next step is to transform solid polylactic acid resin into slender fibers. Bio-based polylactic acid (PLA) fibers are synthetic fibers made from polylactic acid (PLA) through spinning processes (melt spinning, wet spinning, dry spinning, dry-wet spinning, electrospinning, etc.). Currently, there are two mainstream spinning methods: melt spinning and solution spinning, with melt spinning being the most dominant process.
Solution spinning is like "making coffee": the polylactic acid resin is dissolved in a special solvent to form a colloidal solution, which is then extruded from the spinneret and solidified into fibers in the solvent. Fibers produced by this method have better mechanical properties, similar to a well-kneaded dough with excellent toughness. However, the problem is that solvent recovery is difficult and costly. Moreover, solvent volatilization in the spinning workshop requires workers to wear protective clothing, making it suboptimal in terms of both environmental protection and economic efficiency.
 Melt spinning, on the other hand, is like "melting a candle": the polylactic acid resin is directly heated to around 170°C to melt into a molten mass (similar to melted candle wax), which is then extruded as thin streams from the spinneret and solidified into fibers by a blast of cold air. It sounds simple, but there's a great deal of expertise hidden behind it.
Melt spinning, on the other hand, is like "melting a candle": the polylactic acid resin is directly heated to around 170°C to melt into a molten mass (similar to melted candle wax), which is then extruded as thin streams from the spinneret and solidified into fibers by a blast of cold air. It sounds simple, but there's a great deal of expertise hidden behind it.
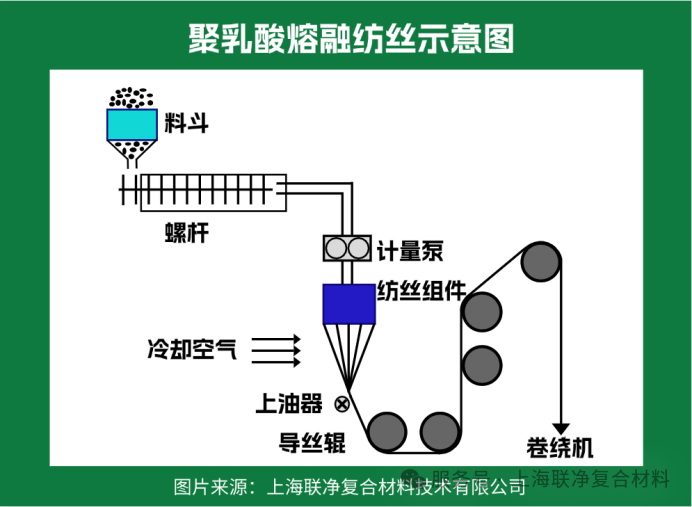 In industrial production, melt spinning is further divided into two methods: the "high-speed one-step spinning process" and the "two-step spinning-stretching process". The one-step process is like an "assembly line operation" — melting, spinning, and stretching are completed in one go. It boasts an efficiency 6 to 15 times that of the two-step process, making it suitable for large-scale production. The two-step process, by contrast, is akin to "meticulous craftsmanship". First, thick filaments are spun, and then they are slowly stretched and thinned. This results in fibers with higher strength, making them ideal for high-end fabrics.
In industrial production, melt spinning is further divided into two methods: the "high-speed one-step spinning process" and the "two-step spinning-stretching process". The one-step process is like an "assembly line operation" — melting, spinning, and stretching are completed in one go. It boasts an efficiency 6 to 15 times that of the two-step process, making it suitable for large-scale production. The two-step process, by contrast, is akin to "meticulous craftsmanship". First, thick filaments are spun, and then they are slowly stretched and thinned. This results in fibers with higher strength, making them ideal for high-end fabrics.
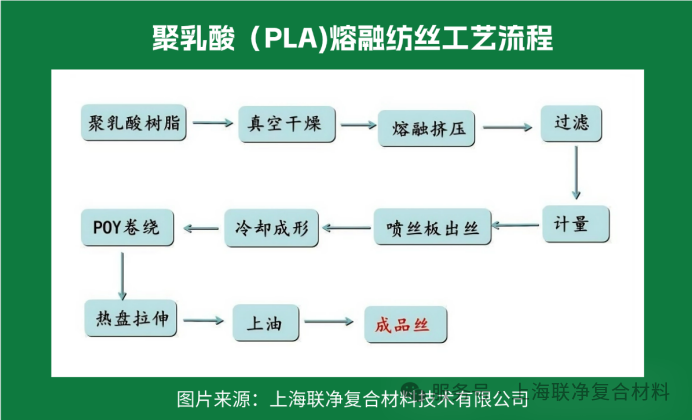 Why has melt spinning become the mainstream? Let's look at this set of data: its pollution emissions are 80% lower than those of solution spinning, the cost is reduced by 30%, and it features a high degree of automation, with a single production line capable of producing several tons of fibers per day. Currently, over 95% of polylactic acid fibers worldwide are "products" of melt spinning.
Secrets Under the Microscope: How Does the Structure of PLA Fibers Determine Their "Extraordinary Properties"?
What does polylactic acid fiber spun by melt spinning look like under a microscope?
Longitudinally, it resembles an uneven "glass rod" with irregular spots and streaks on the surface, similar to ice flowers on windows in winter; its cross-section is approximately circular, with small pits on the surface. These "imperfections" are precisely its advantages — the spots and streaks are traces of a large number of amorphous regions. When exposed to water and bacteria, microorganisms can quickly decompose the fibers along these "channels", eventually turning them into carbon dioxide and water.
Why has melt spinning become the mainstream? Let's look at this set of data: its pollution emissions are 80% lower than those of solution spinning, the cost is reduced by 30%, and it features a high degree of automation, with a single production line capable of producing several tons of fibers per day. Currently, over 95% of polylactic acid fibers worldwide are "products" of melt spinning.
Secrets Under the Microscope: How Does the Structure of PLA Fibers Determine Their "Extraordinary Properties"?
What does polylactic acid fiber spun by melt spinning look like under a microscope?
Longitudinally, it resembles an uneven "glass rod" with irregular spots and streaks on the surface, similar to ice flowers on windows in winter; its cross-section is approximately circular, with small pits on the surface. These "imperfections" are precisely its advantages — the spots and streaks are traces of a large number of amorphous regions. When exposed to water and bacteria, microorganisms can quickly decompose the fibers along these "channels", eventually turning them into carbon dioxide and water.
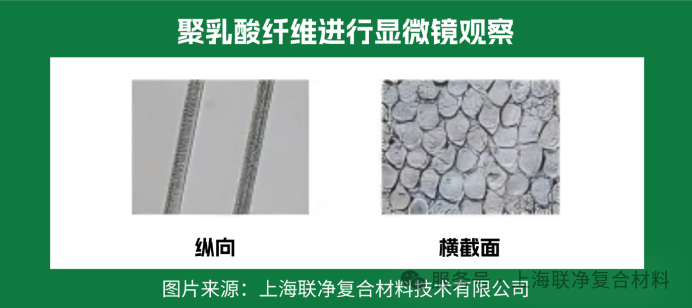 These structures also endow it with "special abilities":
- **Moisture-wicking and breathability**: The grooves and pores on the surface create a "capillary effect", just like plant roots absorbing water. They can quickly draw sweat from the skin to the surface of the fabric for evaporation, making it more comfortable and dry than cotton fabrics in summer.
- **Lightweight and fluffy**: With a density of only 1.27g/cm³, it is 20% lighter than cotton (1.54g/cm³). Coupled with its natural crimp, the quilt made from it is even fluffier than down and remains soft after multiple washes without hardening.
- **Flame-retardant and safe**: It has a limiting oxygen index of 26%, which is higher than that of polyester (21%). It self-extinguishes within 2 minutes after being away from fire, and when burning, it only emits a faint sweet smell without producing toxic gases — this is also why it can be used in children's clothing.
However, it also has "weaknesses": poor heat resistance, as it will shrink when the temperature exceeds 140°C, so it cannot be washed with hot water; weak resistance to acids and alkalis, as it will hydrolyze when exposed to strong alkalis, requiring neutral detergents for laundry. All these are determined by its structure — the crystalline regions tend to "fall apart" at high temperatures, and the ester bonds will break in strong alkalis.
Beyond Environmental Friendliness:
These structures also endow it with "special abilities":
- **Moisture-wicking and breathability**: The grooves and pores on the surface create a "capillary effect", just like plant roots absorbing water. They can quickly draw sweat from the skin to the surface of the fabric for evaporation, making it more comfortable and dry than cotton fabrics in summer.
- **Lightweight and fluffy**: With a density of only 1.27g/cm³, it is 20% lighter than cotton (1.54g/cm³). Coupled with its natural crimp, the quilt made from it is even fluffier than down and remains soft after multiple washes without hardening.
- **Flame-retardant and safe**: It has a limiting oxygen index of 26%, which is higher than that of polyester (21%). It self-extinguishes within 2 minutes after being away from fire, and when burning, it only emits a faint sweet smell without producing toxic gases — this is also why it can be used in children's clothing.
However, it also has "weaknesses": poor heat resistance, as it will shrink when the temperature exceeds 140°C, so it cannot be washed with hot water; weak resistance to acids and alkalis, as it will hydrolyze when exposed to strong alkalis, requiring neutral detergents for laundry. All these are determined by its structure — the crystalline regions tend to "fall apart" at high temperatures, and the ester bonds will break in strong alkalis.
Beyond Environmental Friendliness:
The Application Scenarios of PLA Fibers Have Long Surpassed Your Imagination
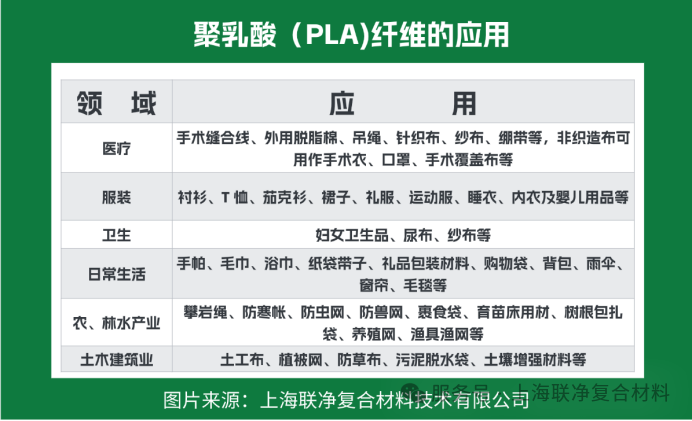 Thanks to its unique structure and properties, the applications of polylactic acid fibers are no longer limited to clothing.
In the medical field, it serves as the optimal material for "absorbable sutures". After closing a wound, these sutures will degrade automatically within 3 to 6 months, eliminating the need for removal. Moreover, lactic acid, the degradation product, is a substance naturally present in the human body, thus avoiding allergic reactions.
Thanks to its unique structure and properties, the applications of polylactic acid fibers are no longer limited to clothing.
In the medical field, it serves as the optimal material for "absorbable sutures". After closing a wound, these sutures will degrade automatically within 3 to 6 months, eliminating the need for removal. Moreover, lactic acid, the degradation product, is a substance naturally present in the human body, thus avoiding allergic reactions.
 In the home textile sector, quilts made from it are "breathable": with a thermal conductivity of 0.0348W/m·℃, which is even lower than that of down, they offer better warmth retention in winter and don't cause stuffiness from sweat when covered in summer. What's more impressive is that it has natural antibacterial properties — the survival rate of mites on it is 60% lower than on cotton fabrics, making it suitable for people with allergies.
In the agricultural field, mulch films made from it, when buried in the soil, will degrade into fertilizers within 2-3 years, eliminating the need for manual cleaning of residual films. Pesticide slow-release bags made from it can release pesticides gradually, reducing pesticide waste.
In the home textile sector, quilts made from it are "breathable": with a thermal conductivity of 0.0348W/m·℃, which is even lower than that of down, they offer better warmth retention in winter and don't cause stuffiness from sweat when covered in summer. What's more impressive is that it has natural antibacterial properties — the survival rate of mites on it is 60% lower than on cotton fabrics, making it suitable for people with allergies.
In the agricultural field, mulch films made from it, when buried in the soil, will degrade into fertilizers within 2-3 years, eliminating the need for manual cleaning of residual films. Pesticide slow-release bags made from it can release pesticides gradually, reducing pesticide waste.
 What is even more noteworthy is its "carbon footprint" — merely 1/10 that of traditional polyester. Producing 1 ton of polylactic acid fiber results in 90% less carbon emissions compared to polyester. Under the "dual carbon" goals, this fiber, which grows from crops and eventually returns to nature, may well become the "protagonist" of the future textile industry.
From the screening of molecular structures, to breakthroughs in melt spinning technology, and to the precise regulation of properties, every step in the development of polylactic acid fiber represents a balance between "environmental protection" and "technology". With future advancements in processes, we may see polylactic acid fibers that are more heat-resistant and tougher. By then, "wearing clothes that can disappear on their own" might become the norm.
What is even more noteworthy is its "carbon footprint" — merely 1/10 that of traditional polyester. Producing 1 ton of polylactic acid fiber results in 90% less carbon emissions compared to polyester. Under the "dual carbon" goals, this fiber, which grows from crops and eventually returns to nature, may well become the "protagonist" of the future textile industry.
From the screening of molecular structures, to breakthroughs in melt spinning technology, and to the precise regulation of properties, every step in the development of polylactic acid fiber represents a balance between "environmental protection" and "technology". With future advancements in processes, we may see polylactic acid fibers that are more heat-resistant and tougher. By then, "wearing clothes that can disappear on their own" might become the norm.