
First author: LIU Feng, ZHANG Chunyang, LV Kejian
Corresponding author: Professor Liu Maochang
Corresponding institution: Xi 'an Jiaotong University
In this study, a CdZnS nanotwin catalyst with atomically dispersed Ru single atoms and clusters was developed to achieve efficient photocatalytic conversion of PLA waste under mild conditions, and to simultaneously produce high value-added chemical pyruvate (PA) and clean energy hydrogen (H2). The results show that Ru single atom generates oxygen free radical through the specific activation of lactic acid α-OH bond, the selectivity of directed PA reaches 96.8%, and Ru clusters as the active site of H2 precipitation achieve a hydrogen production rate of 43.7 μmol h-1 and an apparent quantum efficiency of 83.7% (400 nm). Pilot scale verification shows that the system uses 1.1m x1.1m Fresnel lens to focus sunlight, and can stably output 1191 mL h-1 H2 and 47.27 mmol h-1 PA, providing a large-scale solution for the high-value utilization of plastic waste.
Background introduction
With the increasingly serious global plastic pollution problem, polylactic acid (PLA), as the largest production of biodegradable plastics, has achieved large-scale application. Photocatalysis technology drives REDOX reaction by capturing solar energy through semiconductor materials, which provides a promising green path for the recycling of PLA waste. The technology can convert lactic acid (LA), the hydrolyzed product of PLA, into pyruvate (PA) and other high value-added chemicals, and at the same time precipitate hydrogen (H2), which has both environmental protection and economic value. However, there are two major bottlenecks in traditional photocatalysts: First, the thermodynamic stability of α-C(sp3)-H bond in lactic acid molecules is higher than that of α-OH bond, resulting in the reaction tends to produce by-products and has low PA selectivity; Second, the rapid recombination of photogenerated electron-hole pairs limits the H2 precipitation efficiency. To solve the above problems, single-atom catalysts (SACs) have become a research hotspot because of their unique electronic structure and high ion utilization rate. In particular, Ru single atom can reduce the dissociation energy barrier of α-OH bond by specific adsorption of hydroxyl group, which provides the possibility of directional regulation of reaction path.
Highlights of this article
1. Control of Ru species dispersion state: Controlled loading of Ru single atoms, Ru single atoms and clusters, Ru nanoparticles on the surface of CdZnS nanotwins to achieve selective activation of lactic acid molecules.
2. Single atom-cluster synergistic catalytic mechanism: Ru single atom selectively activates α-OH bond of lactic acid, hydrolyzed product of PLA plastic, to generate PA, and Ru cluster acts as an efficient hydrogen production site, and the two synergistically significantly improve the photocatalytic performance. The selectivity of PA is 96.8%, and the apparent quantum efficiency of H2 is 83.7% (400 nm).
3. Outdoor pilot system verification: Build 1.1m x1.1m solar driven reaction system, PA and H₂ production capacity of 47.27 mmol H-1 and 1191 mL H-1, to achieve large-scale application demonstration of technology. The full life cycle cost assessment shows that the production cost of PA is comparable to that of traditional processes, with both environmental and economic benefits.
Graphic analysis
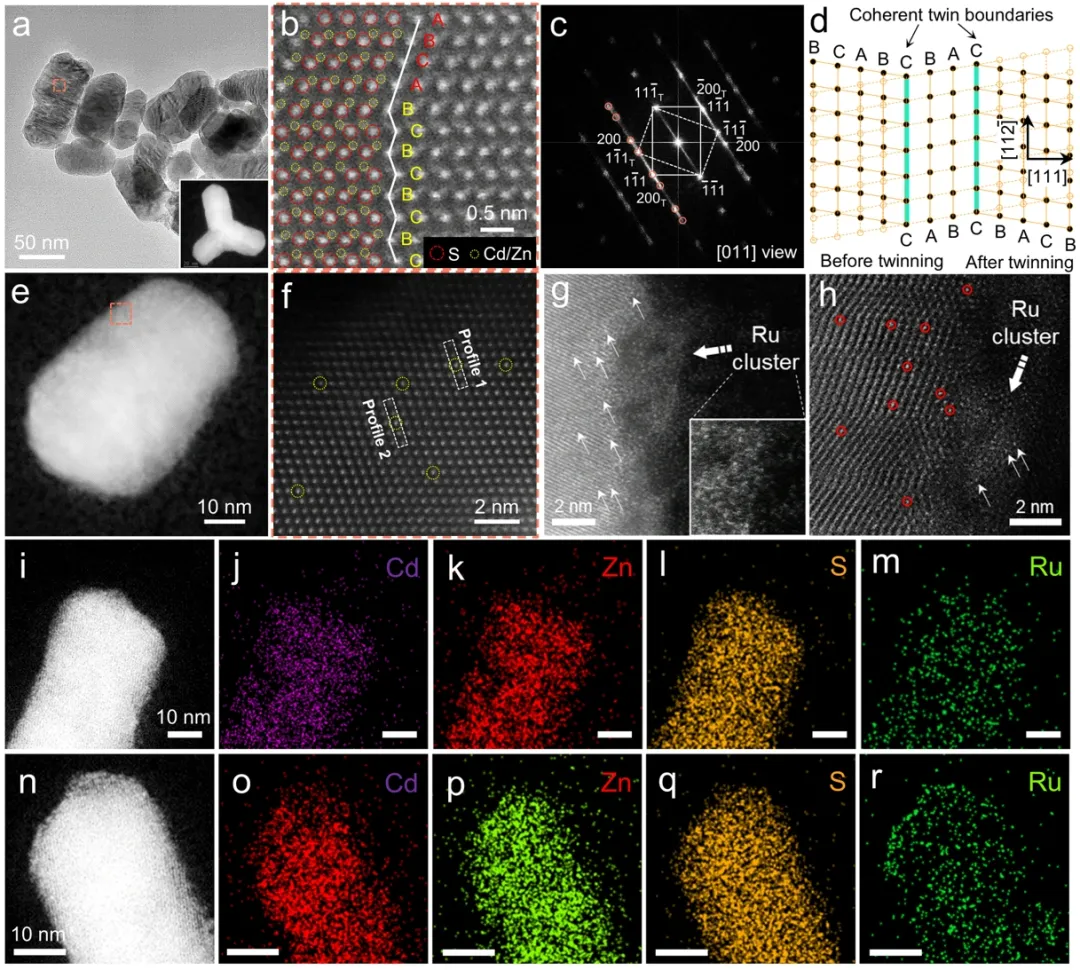
FIG. 1. Catalyst microstructure and Ru dispersion state. CdZnS nanotwins (a) TEM, (b) HRTEM and (c) selected electron diffraction. (d) Schematic diagram of twin structure. (e) TEM, (f) HRTEM and (i-m) element distribution of Ru0.3-CdZnS photocatalyst. (g) TEM, (h) HRTEM and (n-r) distribution of Ru0.6-CdZnS photocatalyst.
Through transmission electron microscopy (TEM), CdZnS nanotwins showed a rod-like structure with a diameter of about 50 nm, and there were high density parallel stacking faults on the surface (FIG. 1a). High resolution electron microscopy (HRTEM) and selected electron diffraction (SAED) confirmed the twin superlattice structure with alternating cubic and hexagonal segments (FIG. 1b-c), and the twin plane arrangement is highly symmetrical (FIG. 1d). This one-dimensional ordered homojunction can promote charge separation through type II band structure. High-angle toroidal dark field scanning electron microscopy (HAADF-STEM) and element surface distribution maps showed that 0.3wt % Ru was loaded on the CdZnS surface as a single atom (FIG. e-f and FIG. i-m). However, 0.6 wt% Ru is uniformly distributed on the surface of CdZnS in the form of co-loading of single atoms and clusters (diameter ≈2 nm) (Figure 1g-h and Figure n-r).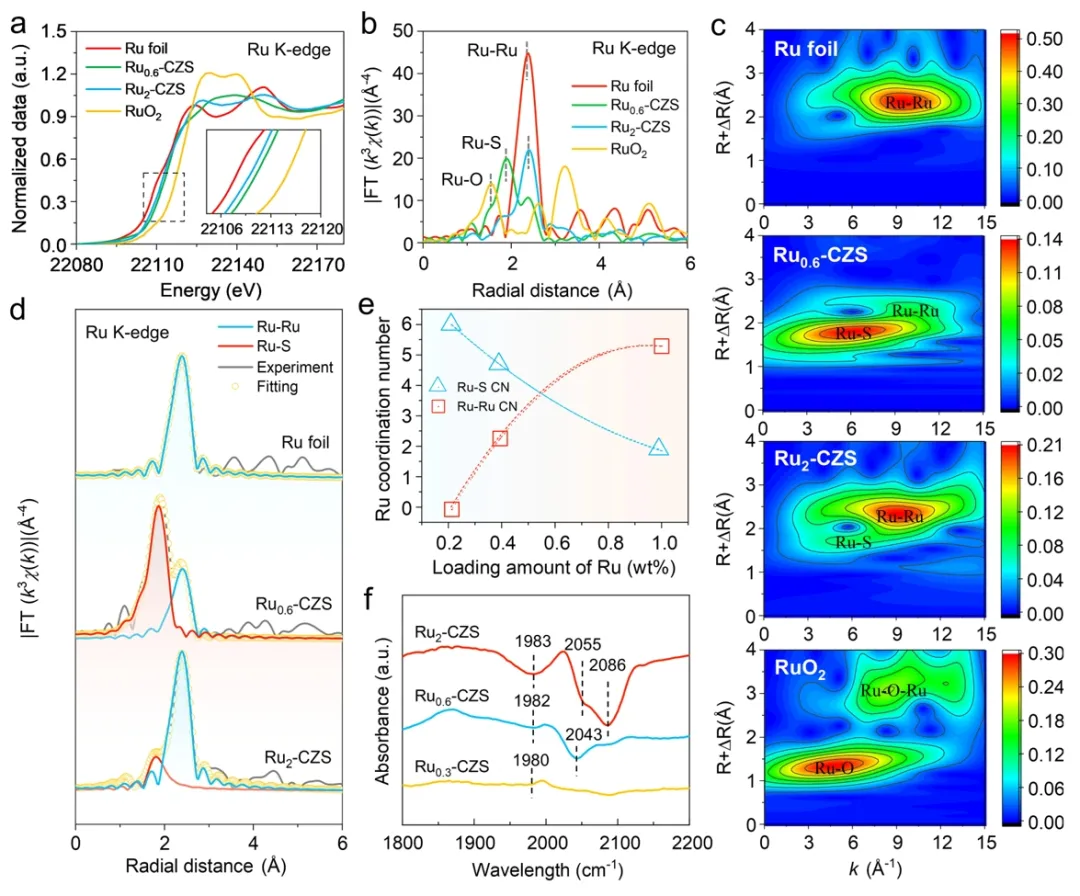 Figure 2. Analysis of the coordination environment of Ru site. (a) Ru0.3-CdZnS, Ru0.6-CdZnS, Ru foil and RuO2 Ru K-side XANES spectrum, (b) Ru K-side FT-EXAFS spectrum, (c) wavelet transform EXAFS signal, (d) Ru-Ru and RU-S signal fitting of FT-EXAFS spectrum. (e) Ru coordination number varies with Ru load. (f) CO-FTIR spectrum.
Figure 2. Analysis of the coordination environment of Ru site. (a) Ru0.3-CdZnS, Ru0.6-CdZnS, Ru foil and RuO2 Ru K-side XANES spectrum, (b) Ru K-side FT-EXAFS spectrum, (c) wavelet transform EXAFS signal, (d) Ru-Ru and RU-S signal fitting of FT-EXAFS spectrum. (e) Ru coordination number varies with Ru load. (f) CO-FTIR spectrum.
The X-ray absorption near Edge structure (XANES) shows that the K-edge absorption threshold of 0.6 wt% Ru is between Ru foil and RuO2 (Figure 2a). X-ray absorption fine structure (XAFS) and wavelet change analysis show that the R monatom is anchored to the CdZnS surface by Ru-S bonds, while the clusters form Ru-Ru bonds (Figure 2b-c). The EXAFS fit showed that 0.6 wt% Ru had both ru-S (1.89A) and ru-Ru (2.39A) bonds (Figure 2d), confirming the synergistic interaction between monatom and cluster. Fourier transform infrared spectroscopy (FTIR) showed that 0.3wt % Ru adsorbed CO in the form of a single atom (1980 cm-1), 0.6wt % Ru showed a cluster adsorption peak (2043 cm-1), and 2wt % Ru formed a nanoparticle adsorption peak (2055 cm-1) (FIG. 2f).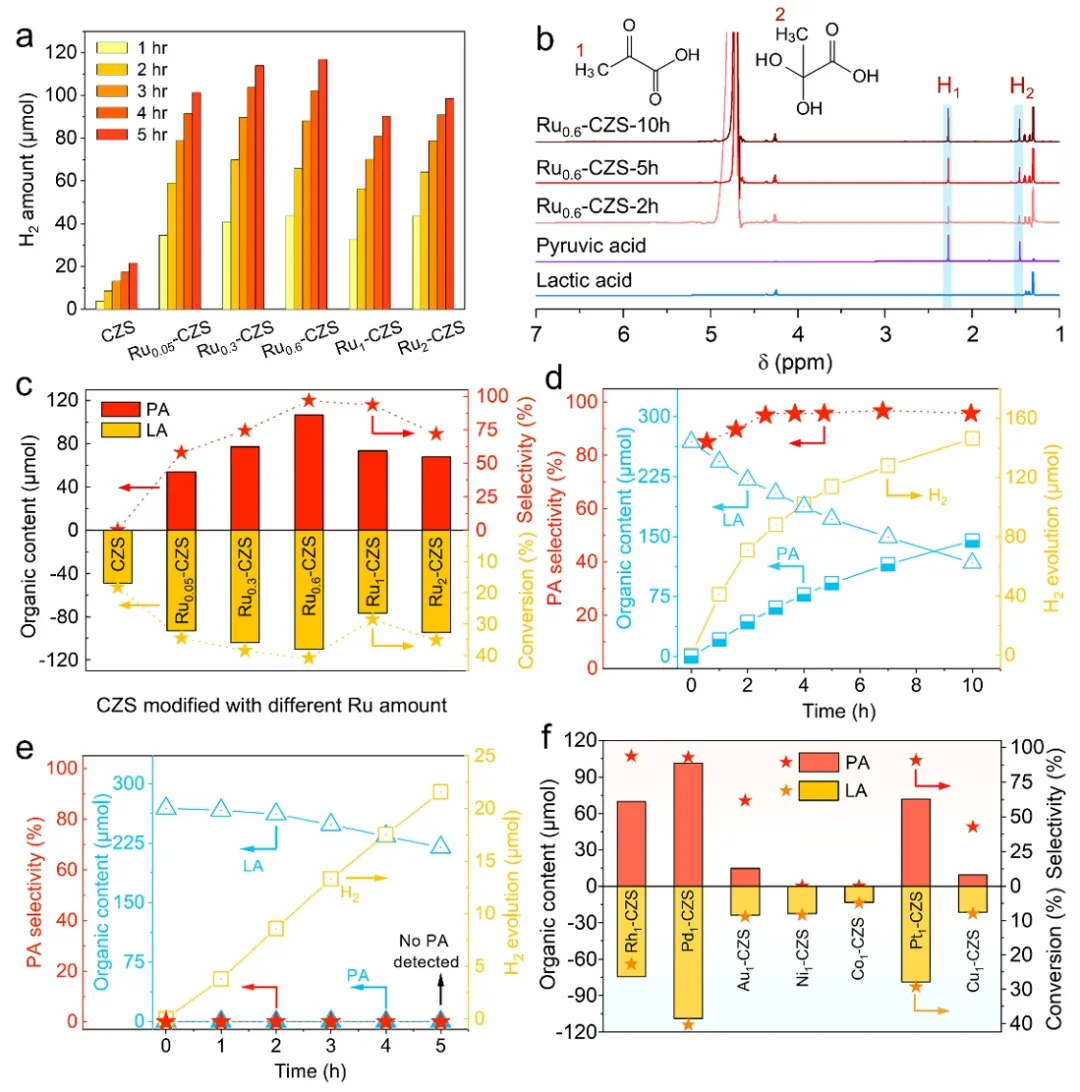 Figure 3. Performance breakthrough of photocatalytic reforming. (a) hydrogen-producing activity of Rux-CdZnS, (b) liquid phase product 1H-NMR, (c) Rux-CdZnS pyruvate yield and selectivity, (d) Ru0.6-CdZnS and (e) CdZnS photoreforming lactic acid performance change with time, (f) CdZnS pyruvate yield and selectivity with different monoatomic modifications.
Figure 3. Performance breakthrough of photocatalytic reforming. (a) hydrogen-producing activity of Rux-CdZnS, (b) liquid phase product 1H-NMR, (c) Rux-CdZnS pyruvate yield and selectivity, (d) Ru0.6-CdZnS and (e) CdZnS photoreforming lactic acid performance change with time, (f) CdZnS pyruvate yield and selectivity with different monoatomic modifications.
Photocatalytic restructuring experiments showed that 0.6 wt% Ru catalyst achieved the highest H2 yield of 43.7 μmol h-1 (FIG. 3a), apparent quantum efficiency (AQE) of 83.7% (400 nm), and PA selectivity of 96.8% (FIG. 3c-d). 1H NMR showed that the -CH3 peak of pyruvate (δ= 2.23ppm) gradually increased with reaction time, while the -OH peak of lactic acid (δ= 4.12ppm) gradually decreased (FIG. 3b). Quantitative analysis by HPLC showed that the pyruvate yield of 0.6 wt% Ru catalyst was 39.6%, while the original CdZnS was 0 (Figure 3d-e). The Ru system exhibits optimal PA selectivity compared to other metals (Figure 3f).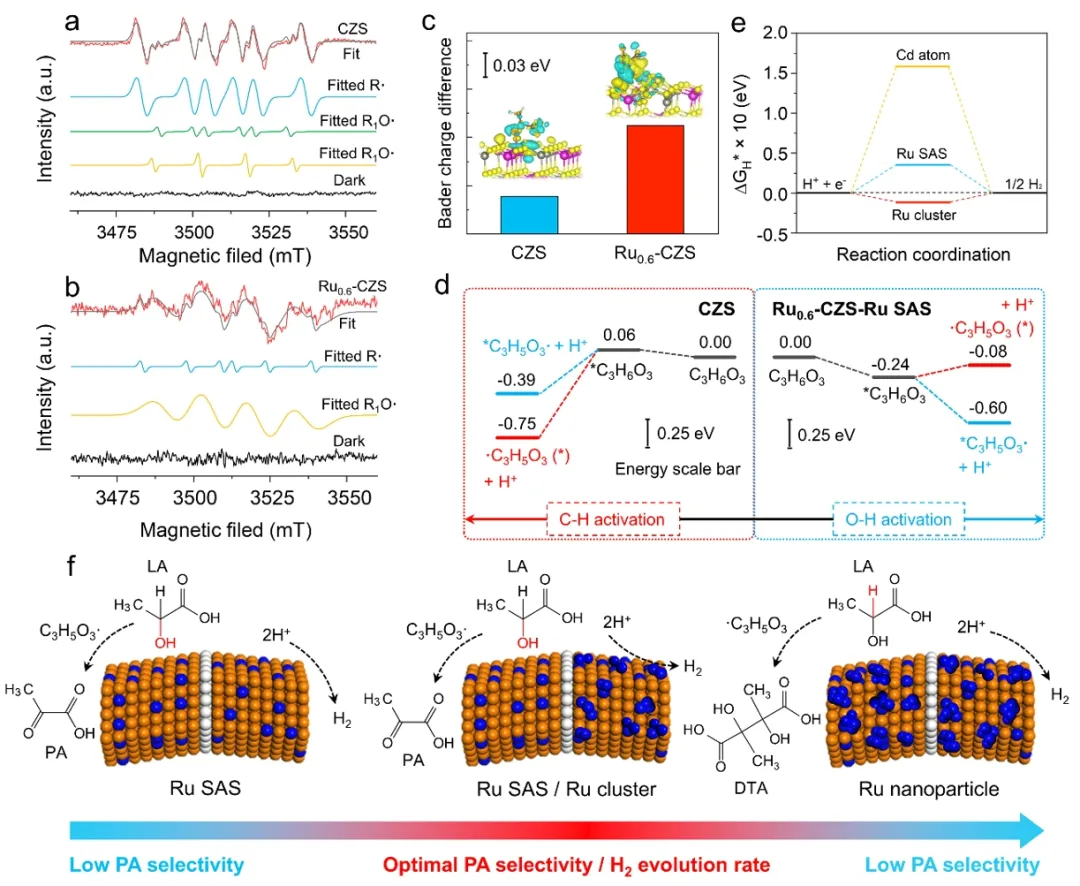 FIG. 4. Mechanism of photoreforming reaction. (a) In situ EPR radical analysis of CdZnS and (b) Ru0.6-CdZnS. (c) Bard charge analysis. (d) Thermodynamic barriers for different reaction paths. (e) Different sites HER-ΔGH*. (f) Schematic diagram of reaction mechanism.
FIG. 4. Mechanism of photoreforming reaction. (a) In situ EPR radical analysis of CdZnS and (b) Ru0.6-CdZnS. (c) Bard charge analysis. (d) Thermodynamic barriers for different reaction paths. (e) Different sites HER-ΔGH*. (f) Schematic diagram of reaction mechanism.
In situ electron paramedical resonance (EPR) and density functional theory (DFT) revealed that Ru monatom enhanced α-OH bond dissociation by electron transfer to form oxygen free radical intermediates leading to pyruvate. CdZnS surface C-H bond dissociation barrier was -0.75eV, which tended to form carbon free radicals. This is followed by C-C coupling to produce 2, 3-dimethyltartaric acid (Figure 4a-d). The calculation of hydrogen adsorption free energy shows that Ru cluster ΔGH* is superior to Ru monatom and promotes H2 precipitation (FIG. 4e). Bader charge analysis confirmed that 0.15e transfer of Ru atom to lactic acid molecule significantly reduced the reaction energy barrier (FIG. 4c). The optimal photopolymerization hydrogen production activity and pyruvate selectivity were achieved through the synergistic interaction of Ru single atom and cluster.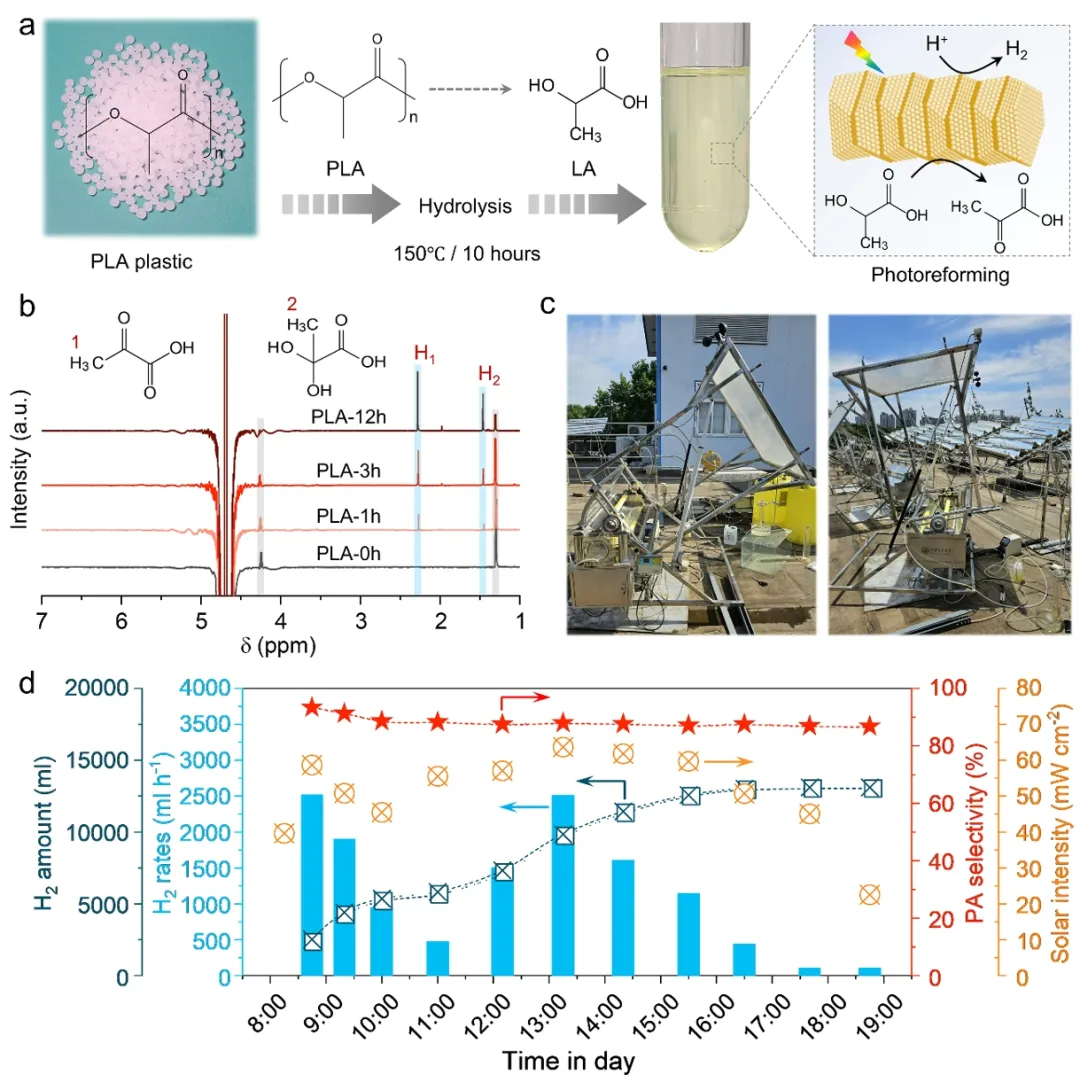 Figure 5. Outdoor pilot system verification. (a) Schematic diagram of PLA plastic photocatalytic reforming process. (b) 1H-NMR transformation of liquid phase products with time. (c) Photos of the tracer concentrating photoreforming system. (d) The effect of PLA plastic photoreforming on the same day.
Figure 5. Outdoor pilot system verification. (a) Schematic diagram of PLA plastic photocatalytic reforming process. (b) 1H-NMR transformation of liquid phase products with time. (c) Photos of the tracer concentrating photoreforming system. (d) The effect of PLA plastic photoreforming on the same day.
The hydrolysate of PLA plastic is directly used in the Ru0.6-CdZnS photocatalytic reforming reaction (FIG. 5a). 1H NMR showed the gradual conversion of PLA hydrolysate lactic acid to pyruvate (Figure 5b). The designed 1.1m x 1.1m Fresnel lens focusing system achieved 12 hours of continuous outdoor sun-driven reaction (FIG. 5c). On the same day, the H2 production rate was 1191 mL h-1, the pyruvate yield was 47.27 mmol h-1, and the pyruvate selectivity exceeded 85% (FIG. 5d). The feasibility of the technology from the laboratory to the actual scenario is verified. Technical and economic analysis shows that the whole life cycle of 50 sets of photoreforming systems can produce 7.27 tons of pyruvate by treating PLA waste, which has market application value.
Summary and prospect
In this study, the controlled and highly selective conversion of pyruvate or 2, 3-dimethyltartaric acid from PLA plastic by photocatalytic reforming was achieved by constructing Ru monoatomic sites and Ru clusters on CdZnS nanotwin catalyst. It is found that CdZnS nanotwins or Ru clusters without Ru monoatomic sites tend to break α-C(sp3)-H bonds in lactic acid molecules and generate 2, 3-dimethyl tartaric acid through carbon radical coupling paths. Atomically dispersed Ru sites stabilized by RU-S bonds can effectively dissociate α-OH bonds and switch products to pyruvate under the mediation of active oxygen species. Through the synergistic interaction of Ru atomic sites and clusters on CdZnS surface, the pyruvate selectivity is optimized by Ru atomic sites, and the hydrogen production activity is improved by Ru clusters, the pyruvate selectivity is up to 96.8%, and the apparent quantum efficiency of hydrogen production is up to 83.7% under 400 nm illumination. The PLA plastic photoreforming reaction was further demonstrated on a large scale in an outdoor installation equipped with a light concentrating system, and achieved a record photocatalytic hydrogen production performance of PLA waste. This work provides a feasible technical path for the conversion of PLA waste into high-value chemicals and green hydrogen energy.








