Chinese name: 聚乳酸
English name: polylactic acid
Polylactic acid (PLA) is a new type of bio-based and renewable biodegradable material, made from starch raw materials extracted from renewable plant resources such as corn and cassava. Starch raw materials are saccharified to obtain glucose, which is then fermented with glucose and certain strains to produce high-purity lactic acid. Finally, polylactic acid of a certain molecular weight is synthesized through chemical synthesis methods. It has excellent biodegradability. After use, it can be completely degraded by microorganisms in nature under specific conditions, eventually generating carbon dioxide and water without polluting the environment. This is very beneficial for environmental protection and is recognized as an environmentally friendly material.
PLA degradation mechanism
The molecular structure formula of polylactic acid (PLA) is shown in the figure. The ester bonds in it are easily hydrolyzed and can be degraded into lactic acid by microorganisms in the body or soil. The final metabolic products are water and carbon dioxide, so it does not cause toxic or side effects to the human body and is very safe to use. Therefore, polylactic acid has been applied in many fields such as medicine and pharmacy, for example, as surgical suturing thread, drug controlled release system, etc.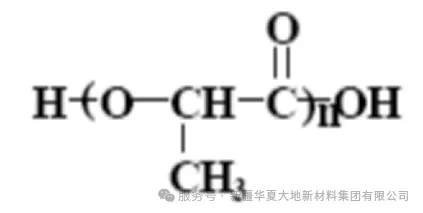
Because lactic acid has optical activity, there are three corresponding polylactic acid types :PDLA, PLLA, and PDLLA(racemic).
PLLA and PDLA are partially crystalline polymers with good mechanical strength and are commonly used as medical sutures and surgical orthopedic materials. Drug controlled-release preparations often adopt PLLA and PDLLA, but PDLLA is more commonly used. The degradation product of PLLA, L-lactic acid, can be completely metabolized by the human body and thus is more competitive than D-PLA.
Degradation in the body
The hydrolysis of PLA is a complex process, mainly including four phenomena: water absorption, cleavage of ester bonds, diffusion of soluble oligomers and decomposition of fragments.
The main mode of degradation: bulk erosion.
When PLA materials are immersed in water-based media or implanted in the human body, water absorption occurs first. The infiltration of water-based media into the polymer matrix causes the relaxation of the polymer molecular chains, the initial hydrolysis of ester bonds begins, the molecular weight decreases, and it gradually degrades into oligomers.
The terminal carboxyl groups of polylactic acid (introduced by polymerization and produced by degradation) play a catalytic role in its hydrolysis. As the degradation proceeds, the amount of terminal carboxyl groups increases and the degradation rate accelerates, thereby generating an autocatalytic phenomenon
Internal degradation is faster than surface degradation, which is attributed to the retention of degradation products with terminal carboxyl groups in the sample, generating a self-accelerating effect.
As the degradation proceeds, there will be more and more carboxyl groups inside the material, accelerating the degradation of the internal material and further increasing the difference between the inside and outside. When the internal materials are completely transformed into soluble oligomers and dissolved in an aqueous medium, a hollow structure with a surface composed of uncompletely degraded polymers will be formed. Further degradation leads to the hydrolysis of oligomers into small molecules, which are finally dissolved in an aqueous medium.
The entire dissolution process is the transformation from a solid insoluble in water to a water-soluble substance.
Macroscopically, the overall structure of the material is destroyed, its volume decreases, it gradually turns into fragments, and finally it is completely dissolved and absorbed or excreted by the human body.
 Microscopically, it is the chemical decomposition of the polymer macromolecular chains, such as the reduction of molecular weight, the breaking of molecular chains and the disruption of side chains, etc., that turns into water-soluble small molecules and enters the body fluids, where they are phagocytosed by cells and transformed and metabolized.
Microscopically, it is the chemical decomposition of the polymer macromolecular chains, such as the reduction of molecular weight, the breaking of molecular chains and the disruption of side chains, etc., that turns into water-soluble small molecules and enters the body fluids, where they are phagocytosed by cells and transformed and metabolized.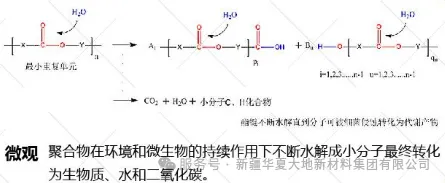
In vitro degradation
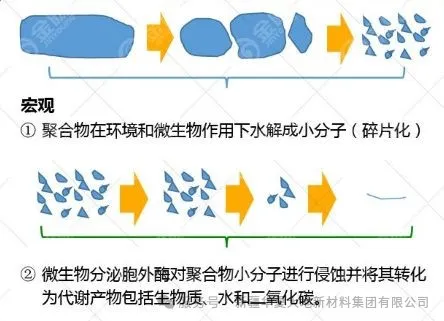
The decomposition of polylactic acid has two stages: decomposition through hydrolysis reaction and then decomposition by microorganisms.
In the natural environment, hydrolysis occurs first, and oligomers are formed through the hydrolysis of unstable ester bonds on the main chain. Then, microorganisms enter the tissue and decompose it into carbon dioxide and water. Under composting conditions (high temperature and high humidity), hydrolysis reactions can be easily completed and the decomposition rate is also relatively fast. In an environment where hydrolysis reactions are not likely to occur, the decomposition process is gradual.
Microorganisms are ubiquitous in nature, and polylactic acid can be degraded by a variety of microorganisms. Such as Candida fusarium, Penicillium, humus bacteria, etc.
The degradation of polylactic acid of different isomorphisms by different bacteria is different. The research results show that both Candida fusarium and Penicillium can completely absorb D-L lactic acid, and some can also absorb soluble polylactic acid oligomers.
Influencing factors of degradation
1) pH value

Both acids and bases can catalyze the hydrolysis of PLA. The rate of degradation of polylactic acid under alkaline conditions is greater than that under acidic conditions and greater than that under neutral conditions.
2) Crystallinity

The degradation process always proceeds from the amorphous region to the crystalline region. This is because the molecular chain segments in the crystalline region are closely packed, making it difficult for water to penetrate. It first penetrates into the amorphous zone, causing the ester bond to break. Only when most of the amorphous zone has been degraded does it start to degrade from the edge to the center of the crystalline zone. During the hydrolysis process in the amorphous region, low-molecular-weight substances with regular structures are generated, and the crystallinity increases, which delays the further hydrolysis process
3) Molecular weight and its distribution
The molecular weight is inversely proportional to the degradation rate. The larger the molecular weight, the tighter the structure of the polymer, and the less likely the ester bonds inside are to break. Moreover, the larger the molecular weight, the longer the chain segments obtained through degradation, which is less soluble in water and generates less water and hydrogen cations, causing the pH value to drop more slowly. This is also one of the reasons why its degradation rate is lower than that of low-molecular-weight polylactic acid.
For polymers with the same average molecular weight, the wider the molecular weight distribution, the faster the degradation rate. This is because the polymers with smaller molecular weights decompose first, and then the environmental pH value changes from neutral to acidic, thereby accelerating the degradation rate.
4) The influence of the regularity of the vertical structure
Under alkaline conditions, the degradation rate is PDLA (PLLA)<P (LDL)A<PDLLA
Due to the fact that the methyl groups in PDLLA are in an isomorphic or random constitutive state, the absorption rate of water is relatively fast, and thus the degradation is relatively fast. For PLLA and PDLA, hydrolysis is divided into two stages: In the first stage, water molecules diffuse into the amorphous region and then hydrolysis occurs; The second stage is the hydrolysis of the crystal region, which is relatively slow.
5) Enzyme
The main chain of polylactic acid contains ester bonds, which can be accelerated for degradation by esterase.
Such as Rhizopus








