With the intensification of environmental pollution and the increasing scarcity of water resources, the development of green, safe and sustainable water treatment technologies has become a global research hotspot. Nanofiltration membranes, as an important component of efficient separation technologies, have been widely applied in industrial wastewater treatment and resource recovery in recent years. However, traditional nanofiltration membranes usually rely on petroleum-based raw materials, and the preparation process involves toxic and harmful solvents, making the membrane materials difficult to degrade, which severely restricts their environmental adaptability and sustainable development potential. Therefore, how to construct new membrane materials that conform to the principles of green chemistry throughout the entire process from raw material selection, preparation technology to waste degradation has become a core challenge that urgently needs to be addressed in the field of membrane science.
Recently, Professor Shao Lu from Harbin Institute of Technology, in collaboration with Professor Wang Huanting from Monash University in Australia and other researchers, based on the concept of green chemistry, established a brand-new green and sustainable nanofiltration membrane (SNFM). Using polylactic acid (PLA) as the support layer, they employed low-hazard chemicals such as xylitol, dopamine, and oleic acid, and constructed a unique wave-like nano-selective separation layer through an interfacial polymerization method. This approach not only avoided the use of traditional hazardous solvents but also enabled the membrane to be degraded by microorganisms in natural soil environments, achieving full life-cycle membrane water purification.
This research not only fills the gap in sustainable nanofiltration membrane technology in the field of both degradation and high performance, but also provides a new paradigm for the green design and industrial application of membrane materials. The related research results, titled "Sustainable nanofiltration membranes enable ultrafast water purification", were published in "Nature Water" on September 17th.

Studies have shown that the green and sustainable nanofiltration membrane exhibits an extremely high water flux (up to 100.7 Lm-2h-1bar-1) during the treatment of dye wastewater, with a retention rate of 99.9% for organic active molecules such as Congo Red. Its separation performance is significantly superior to that of current commercial membranes and advanced research membranes. More importantly, this membrane can be completely degraded by typical microorganisms in an enzymatic environment and natural soil. Within 6 months, the degradation rate reaches over 90%, and the degradation products are non-toxic to the environment and microorganisms, truly achieving a "green source - efficient use - degradable end" full life-cycle sustainable membrane technology.
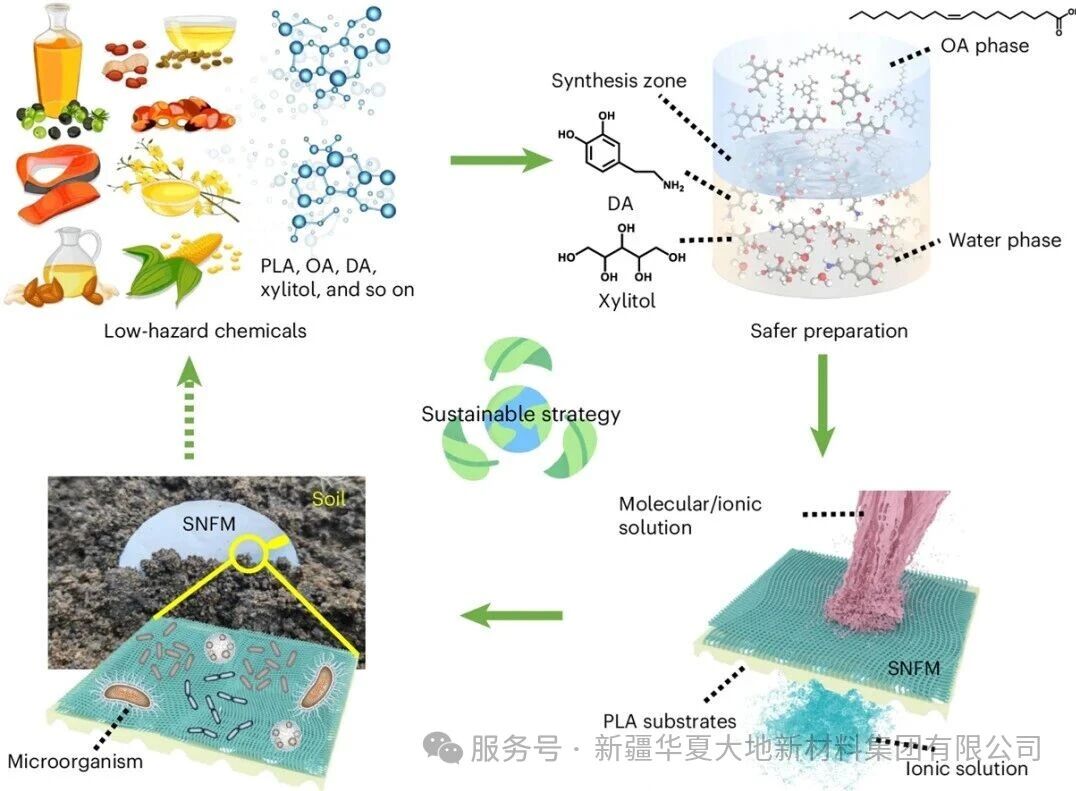
Figure 1 Schematic diagram of the sustainability strategy for sustainable nanofiltration membranes (SNFM)
Figure 1 presents a schematic diagram of the sustainability strategy for the sustainable nanofiltration membrane (SNFM). This membrane is synthesized through a safer preparation process using low-hazard chemicals (including polylactic acid (PLA), xylitol, dopamine (DA), and oleic acid (OA)), and exhibits excellent capabilities for screening restricted molecules and ions. The schematic diagram depicts its preparation process: the aqueous phase of molecules/ion and the oil phase undergo interfacial polymerization on the PLA substrate to form the SNFM. More importantly, this figure also illustrates that the SNFM can be biodegraded by soil microorganisms after its lifespan ends, achieving a natural cycle, highlighting its full life-cycle sustainability from preparation, efficient water treatment (energy efficiency application) to final degradation.
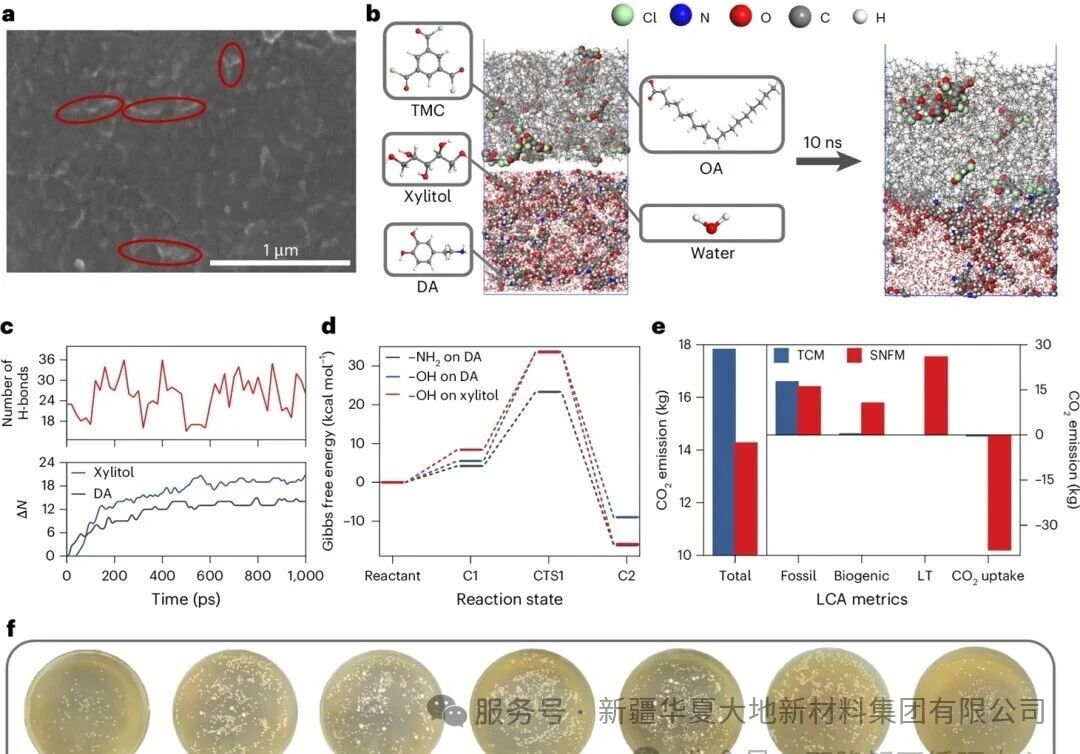
Figure 2: The preparation process of SNFM and the hazard analysis of its raw materials
Figure 2 illustrates the preparation process of SNFM and the hazard analysis of its raw materials. Figure a shows the scanning electron microscope image of the surface of SNFM, presenting a dense and irregular striped structure. Figure b demonstrates the snapshot of the synthesis process of SNFM through molecular dynamics simulation. Figure c reveals the number of hydrogen bonds between DA and xylitol molecules and the curve of the increase in the number at the interface, explaining the mechanism by which the two molecules, through strong hydrogen bond interactions, diffuse towards the interface and form a dense selective layer. Figure d shows the Gibbs free energy of the reaction between DA, xylitol and TMC, indicating the existence of a competitive reaction that leads to the formation of an irregular striped structure. Figure e compares the CO₂ emissions of TCM and SNFM systems through life cycle assessment, indicating that the SNFM system has a lower overall CO₂ emission and becomes a powerful carbon sink due to the use of fast-growing biomass and the implementation of land management practices that increase carbon removal. Figure f visually compares the toxicity of the blank group and the SNFM raw material group through an E. coli growth experiment, confirming that the raw materials used in SNFM are harmless and even promote bacterial growth, far superior to the strong toxicity of the TCM raw materials.
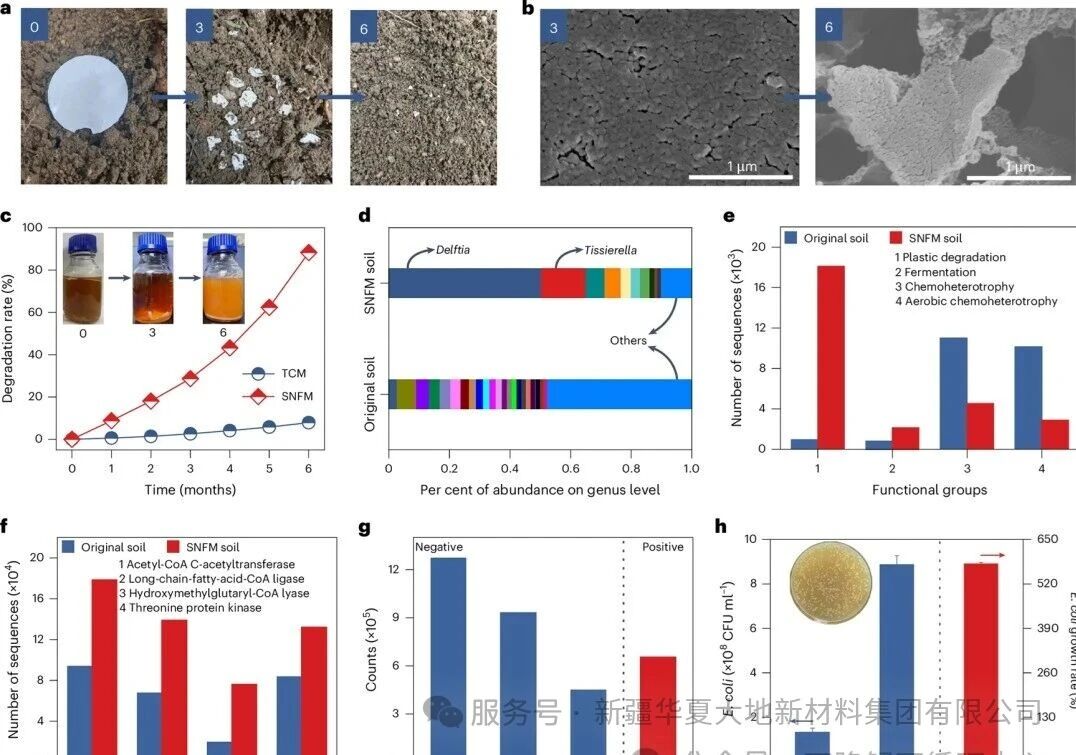 Figure 3 The degradation mechanism of SNFM and its products
Figure 3 The degradation mechanism of SNFM and its products
Figure 3 illustrates the degradation mechanism of SNFM and its products. Figure a shows the morphological changes of SNFM after being buried in natural soil for 0, 3, and 6 months, confirming its gradual degradation. Figure b's scanning electron microscope image reveals that pores and cracks gradually appear on the surface of SNFM after being degraded by soil microorganisms for 3 and 6 months. Figure c quantitatively demonstrates that the degradation rate of SNFM based on soil microorganisms reaches up to 90% after 6 months, which is much higher than that of TCM. Figures d and e indicate through microbial community analysis that SNFM selects specific degradation bacterial genera (such as Delftia and Tissierella) as carbon sources and enriches the bacterial communities with plastic degradation and fermentation functions. Figure f shows that the abundance of fermentation-related enzymes (such as acetyl-CoA-related enzymes) significantly increases in the SNFM soil community, indicating that fat fermentation is the main degradation pathway. Figures g and h determine the degradation products as lactic acid, PLA oligomers, and catechol through liquid chromatography-mass spectrometry analysis, and prove through Escherichia coli toxicity tests that these products are low in hazard and can even significantly promote bacterial growth.
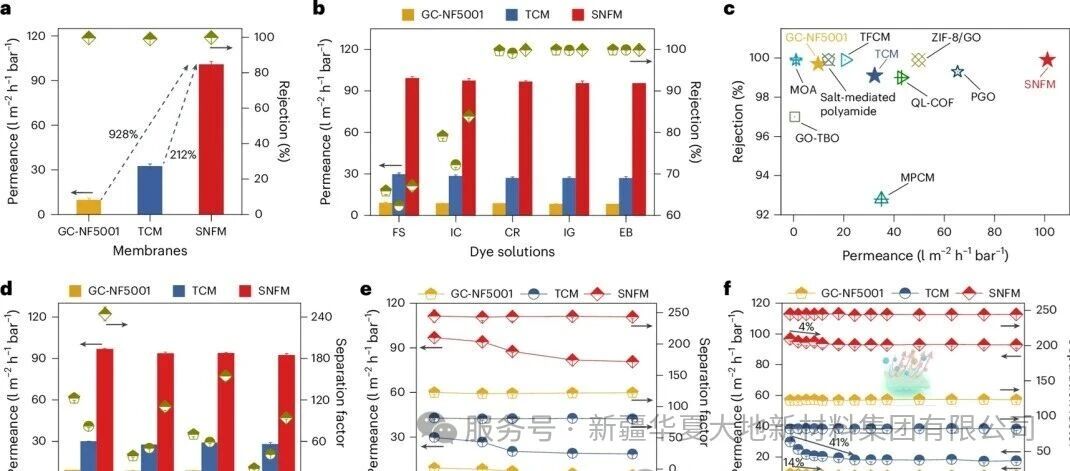
Figure 4: Separation Performance of SNFM
Figure 4 presents a comparison of the separation performance of SNFM. Figure a shows that the pure water flux of SNFM is as high as 100.7 L m⁻² h⁻¹ bar⁻¹, which is 212% and 928% higher than that of the control TCM and commercial GC-NF5001 membranes respectively. At the same time, the retention rate for Congo Red dye is as high as 99.9%. Figure b indicates that SNFM exhibits high solution flux and excellent retention rate of over 99% when treating different active dye molecules (such as CR, IG, EB). The performance comparison diagram in Figure c further demonstrates that the performance of SNFM is superior to many of the current most advanced membrane materials. Figure d shows that SNFM has high solution flux and separation factor in the dye/salt mixed solution, especially a higher separation factor in the monovalent anion system, which is attributed to the Tangnan effect caused by the negative charges on the membrane surface. Figure e indicates that SNFM maintains high and stable flux and separation factor under different Congo Red concentrations. Figure f proves that SNFM can maintain high flux and persistent high separation factor during the continuous operation of 14 days in treating CR/NaCl solution, which is attributed to its excellent anti-pollution ability. The surface hydrophilicity can form a hydration layer to inhibit pollutant adsorption, and the schematic diagram vividly demonstrates this mechanism.
Conclusion Outlook
Through a systematic design strategy from raw materials to collaborative preparation and synthesis methods, this research successfully developed an efficient water treatment sustainable nanofiltration membrane (SNFM) with a more environmentally friendly life cycle. Life Cycle Assessment (LCA) and experiments on E. coli survival demonstrated that compared to traditional composite membranes (TCM), the preparation process of SNFM is safer, and the raw materials used have lower environmental impact and hazards, specifically manifested as lower CO₂ emissions and promoting E. coli growth. Enzyme degradation and soil degradation analysis indicated that SNFM can be degraded by proteinase K within 5 days and by Delftia and Tissierella genus microorganisms within 6 months, while commercial GC-NF5001 membrane and TCM did not show degradability, which proved the sustainability of SNFM. More importantly, SNFM demonstrated a high water flux of 100.7 L m⁻² h⁻¹ bar⁻¹ (928% higher than GC-NF5001 and 212% higher than TCM), excellent retention rate, high molecular/ion separation factor, outstanding anti-pollution ability and operational stability, and its performance was superior to commercial and the current most advanced membranes.
Overall, the article presents a solution for green nanofiltration membranes throughout the entire life cycle. It has achieved breakthroughs in the "performance - sustainability - degradability" aspects, and holds significant milestone importance for future sustainable water treatment and membrane separation technologies.








