When you are shopping at the supermarket and pick up a plastic bottle, or when you order takeout and see the plastic packaging, have you ever wondered: "The same is 'plastic' - why does some of it gradually 'disappear' in the environment after use, while others remain in the soil and the ocean for hundreds of years?"
The petroleum-based plastics we often come into contact with, such as PET (material for mineral water bottles), PP (material for takeout food containers), and PE (material for plastic bags), and the biodegradable materials like PLA (polylactic acid, biodegradable straws), PHA (polyhydroxyalkanoates, biodegradable packaging), and PBAT (biodegradable mulch film), although they may have similar appearances, actually have very different "fates".
 Let's establish a core point first: The essence of degradation is "breaking the long chain". No matter what type of plastic it is, fundamentally, it is a "long chain" (polymer) formed by countless small molecules (monomers) linked together through chemical bonds. For example, PE is a long chain of polyethylene formed by the connection of ethylene molecules through chemical bonds; PLA is a polymer chain formed by the connection of lactic acid molecules.
Let's establish a core point first: The essence of degradation is "breaking the long chain". No matter what type of plastic it is, fundamentally, it is a "long chain" (polymer) formed by countless small molecules (monomers) linked together through chemical bonds. For example, PE is a long chain of polyethylene formed by the connection of ethylene molecules through chemical bonds; PLA is a polymer chain formed by the connection of lactic acid molecules.
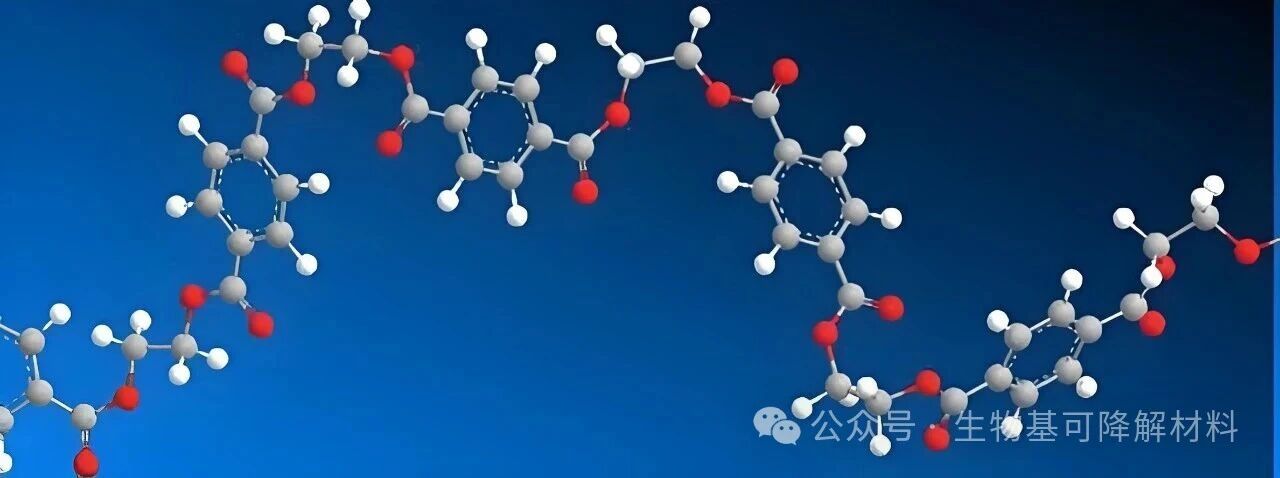
The entire process of "degradation" can be simply described as follows: this "long chain" is gradually broken down in the environment: first, it breaks into shorter "medium molecular fragments", then it disintegrates into "small molecules" that can be absorbed by microorganisms, and finally, it is metabolized by microorganisms into CO₂ and water, returning completely to nature. Whether plastic can degrade and how quickly it degrades mainly depend on two issues: first, are the "chemical bonds" connecting the small molecules strong enough to be easily broken; second, can water, enzymes, and microorganisms in the environment smoothly come into contact with these chemical bonds and "take action"? Petroleum-based plastics (PET, PP, PE): From chemical bonds to structure, they all have "anti-degradation designs". Why are PET, PP, and PE, these petroleum-based plastics, so "stubborn"? It's not that they "intentionally resist", but every characteristic from the molecule to the structure makes degradation extremely difficult. 1. The first hurdle: The chemical bonds are too "strong", and natural energy cannot break through the "framework" of the plastic. The core framework of petroleum-based plastics is composed of chemical bonds, and the core framework of PET, PP, and PE is the carbon-carbon single bond (C-C bond) - this is a naturally "durably resistant" chemical bond: The bond energy is extremely high: The bond energy of the C-C bond is approximately 347 kJ/mol. To break it, extreme conditions such as high temperature and strong oxidants are required to provide energy;
while the normal temperature and pressure in the natural environment, as well as the enzymes secreted by microorganisms, are far from reaching the "energy threshold" required to break it. No "identification markers": The core "tool" for biological degradation is enzymes (the catalysts secreted by microorganisms), but enzymes are very "selective", only recognizing "polar functional groups" on the molecular chain (such as atoms with positive and negative charges, like the oxygen atom in ester bonds). However, in the molecular chains of PP and PE, there are all non-charged carbons and hydrogens, like a smooth "plastic rope", and enzymes simply cannot find a "target point".
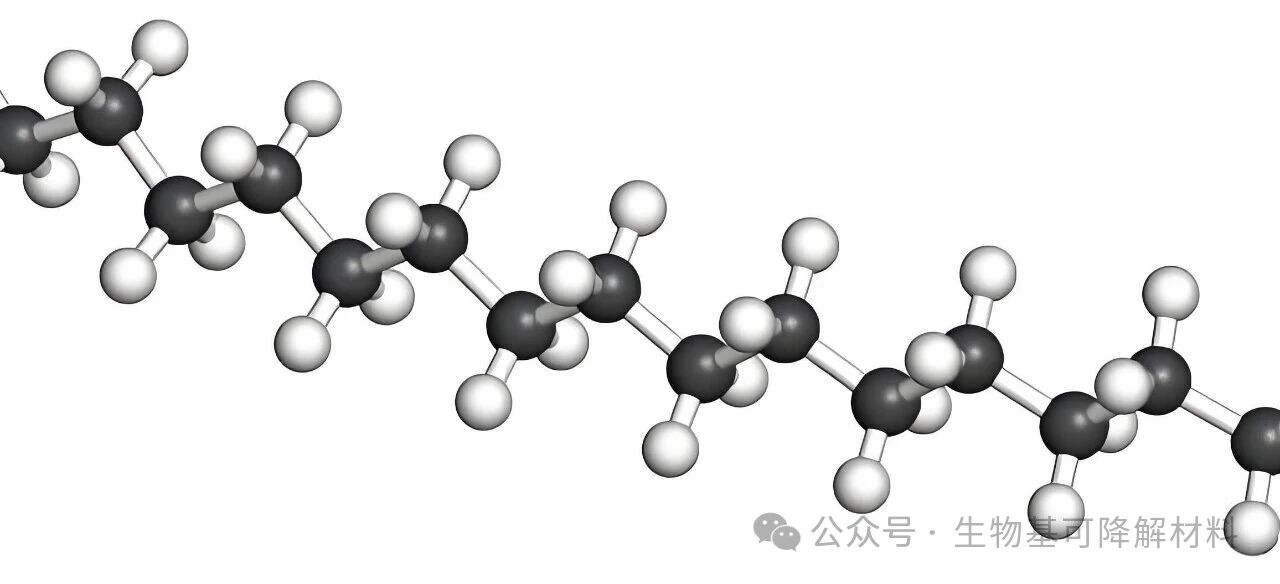
Even for PET, a plastic with a small amount of ester bonds (-CO-O-), the ester bonds are merely "ornaments" - its main chain is still composed of dense C-C bonds, and the ester bonds are firmly "encapsulated" inside, so water and enzymes cannot come into contact with them; moreover, the "stability" of the C-C bonds makes it difficult for the entire molecular chain to break.
2. The second hurdle: The molecular chains are "too densely packed", and water and enzymes "cannot enter" Apart from the strong chemical bonds, petroleum-based plastics also have a particularly "dense" molecular structure. The molecular chains of PP and PE are "non-polar" (without positive or negative charge groups), and these chains will be tightly "stacked" together like dried noodles. In simple terms, there are no voids inside the material, and the density is extremely high. Water and enzymes in the environment, even if they want to attack the chemical bonds, simply cannot penetrate into the material interior; they can only "rotate" on the surface. Even if a few C-C bonds occasionally break, it is only a slight surface wear and cannot break the entire "long chain" into small molecules.
3. The third hurdle: Completely "unhydrophilic" and "zero interaction" with the environment Water is an important "helper" for degradation - many degradation reactions (such as ester bond hydrolysis) require water participation, but PP, PE, and PET are typical "hydrophobic materials". Just like oil and water never mix, these plastics actively "repel" water, and even the surface is difficult to be wetted by water. Without the participation of water, even if enzymes want to catalyze the reaction, it is like "a good cook lacking ingredients", and cannot initiate the chain-breaking process.
4. The fourth hurdle: Microorganisms "do not recognize" and no one "helps" for metabolism Microbial degradation of plastics is essentially "treating the plastic as food". However, microorganisms have strict "recipes" for eating - they only recognize the "molecular structures" common in nature, such as ester bonds in plant fibers and hydroxyl groups in starch, which they come into contact with every day. And petroleum-based plastics are artificially synthesized, and natural microorganisms have never seen molecular chains dominated by C-C bonds, there are no corresponding enzymes to "digest" them, nor will they treat it as "food", so they will not actively participate in degradation. These four hurdles combined make petroleum-based plastics "stubborn molecules" in the environment - even after decades or centuries, they can only slowly break down into "microplastics", but cannot truly disappear. Biobased degradable materials (PLA, PHA, PBAT): From chemical bonds to structure, all are "degradable designs" While PLA, PHA, and PBAT, these biobased degradable materials, can "disappear" in the environment, precisely because every characteristic from molecule to structure of them is "cooperating" with the degradation process.
 1. Core Advantage: Chemical bonds have "weak points" that can be easily broken. These degradable materials have a common "key structure" - ester bonds (-CO-O-). This is the key reason why they can be degraded: the bond energy is low and easy to break. The bond energy of ester bonds is approximately 314 kJ/mol, much lower than that of C-C bonds. Moreover, the oxygen atom in ester bonds has a strong electronegativity, which reduces the electron cloud density of adjacent chemical bonds, equivalent to leaving a "weak link" for the "long chain" - without extreme conditions, water or enzymes can easily "push" it. Enzymes can precisely "recognize": the polar oxygen atom in ester bonds, just like attaching a "degradable label" to the molecular chain. Microorganisms in soil or water bodies will immediately "recognize" this label and secrete specific "lipase" and "esterase" - the active centers of these enzymes can precisely "grab" the ester bonds, and break the bonds at normal temperature and pressure without the need for extreme conditions.
1. Core Advantage: Chemical bonds have "weak points" that can be easily broken. These degradable materials have a common "key structure" - ester bonds (-CO-O-). This is the key reason why they can be degraded: the bond energy is low and easy to break. The bond energy of ester bonds is approximately 314 kJ/mol, much lower than that of C-C bonds. Moreover, the oxygen atom in ester bonds has a strong electronegativity, which reduces the electron cloud density of adjacent chemical bonds, equivalent to leaving a "weak link" for the "long chain" - without extreme conditions, water or enzymes can easily "push" it. Enzymes can precisely "recognize": the polar oxygen atom in ester bonds, just like attaching a "degradable label" to the molecular chain. Microorganisms in soil or water bodies will immediately "recognize" this label and secrete specific "lipase" and "esterase" - the active centers of these enzymes can precisely "grab" the ester bonds, and break the bonds at normal temperature and pressure without the need for extreme conditions.
2. Structural Assistance: The molecular chains have "gaps", and water and enzymes can "enter" easily. Unlike petroleum-based plastics, the molecular chains of degradable materials contain polar ester bonds, and there will be a weak "repulsive force" between the chains, not as dense as PP or PE. The material has tiny "voids" inside. This gives water and enzymes an opportunity: they can easily penetrate the material and come into contact with every ester bond, initiating the chain-breaking reaction from the inside out, rather than just "circulating" on the surface.
3. Environmental Adaptability: Self-contained "hydrophilic groups" and "friendly interaction" with water. The oxygen atom in ester bonds is not only the "recognition label" for enzymes, but also a "hydrophilic hook" - although these degradable materials do not "disintegrate" when exposed to water, their surfaces can be wetted by water, which can smoothly enter the material and participate in the "hydrolysis reaction" of ester bonds (using water molecules to break the ester bonds). With water as the "medium", the catalytic efficiency of enzymes will be greatly improved, and the chain-breaking process will be faster.
4. Microbial Preference: Natural "food", active metabolism. What's more, the molecular structure of these degradable materials is already a "familiar taste" for microorganisms:
PLA comes from plant starches such as corn and sugar cane, and after hydrolysis, it can obtain lactic acid - this is a common small molecule in nature;
PHA is more direct, it is the "energy reserve substance" synthesized by certain bacteria (such as Rosellonella bacteria) during fermentation, like the "fat" of bacteria;
The molecular structure of PBAT contains a large number of ester bonds similar to natural oils.
Therefore, when these degradable materials enter the environment, microorganisms will immediately "recognize": "This is edible nutrition!" They will actively secrete enzymes to "digest" the ester bonds, and the small molecules (dialycic acids, dialcohols) after chain-breaking will be quickly metabolized into CO₂ and water, and completely integrated into nature. In one sentence: Degradation is the result of "multiple factors cooperating".
The difficulty of degrading petroleum-based plastics is caused by "strong C-C bonds + dense structure + hydrophobic characteristics + microorganisms not recognizing", while the ability to degrade biobased degradable materials is the result of "easily broken ester bonds + loose structure + hydrophilic characteristics + microorganisms being able to utilize". Chemical bonds are the "basic threshold", but the material structure, the way of interaction with the environment, and the adaptability to microorganisms are the key factors determining whether degradation can proceed smoothly. Next time you see "degradable plastics", you will know that it is not relying on "a single gimmick", but from the molecular level to environmental adaptation, it has achieved "peaceful coexistence" with nature - this is the core reason why it can truly "disappear".