The core advantages of biodegradable polyester materials such as polylactic acid (PLA), polycaprolactone (PCL), and poly(lactide-co-glycolide) (PLGA) lie in their outstanding and customizable properties. These materials not only possess excellent biocompatibility with the human body, preventing persistent inflammatory responses and eventually degrading into non-toxic products that can be metabolized by the body, but also have precisely controllable degradation cycles and mechanical properties that match the requirements of tissues. Moreover, they exhibit excellent processing adaptability. All of these characteristics are deeply influenced by the chemical and physical properties of the materials themselves, including molecular weight, hydrophilicity/hydrophobicity, surface charge, and degradation mechanism, etc. Therefore, they can be precisely customized through molecular design to meet the needs of specific biomedical applications.
01. Biocompatibility
The safety of degradable polyester materials is rooted in their outstanding biocompatibility. This is demonstrated by the fact that both the material itself and its degradation products can coexist harmoniously with the biological system: when in contact with living tissues, blood, or body fluids, the material can effectively perform its designed functions without causing harmful local or systemic reactions; its degradation products can be safely absorbed or excreted by the body. For example, the final degradation product of polylactic acid (PLA) is lactic acid, which is a naturally occurring metabolic intermediate in the human body and can smoothly enter the tricarboxylic acid cycle (TCA cycle) for complete metabolism, ultimately generating carbon dioxide and water, and being excreted from the body through physiological pathways such as respiration and urination, thus achieving complete clearance within the body.
02. Adjustable Degradability
The key advantage of biodegradable polyester materials lies in the fact that their degradation behavior can be precisely controlled to match the intrinsic rhythm of tissue regeneration. When functioning as a "temporary scaffold" in the body, their degradation (including both surface erosion and bulk erosion) is not merely a physical disappearance, but also actively regulates the repair microenvironment and the regeneration process. By adjusting parameters such as monomer composition, molecular weight, and crystallinity [2], the retention time of the material in the body can be precisely designed to range from several weeks to several years, thus meeting the different tissue regeneration requirements from short-term skin filling (3-6 months) to long-term deep support (12-24 months).

03. Adjustable mechanical properties
The clinical success of biodegradable materials hinges on whether their mechanical properties can achieve dynamic adaptation with the target tissue. Unlike traditional inert implants, they must provide timely and appropriate mechanical support during the tissue repair period and gradually withdraw as the new tissue grows. This requires the modulus, strength, and degradation behavior of the materials to have the designability of "time-space-force" in unity.
By employing molecular engineering techniques (such as adjusting the ratio of PLA/PCL/PGA copolymers, chain structure, and crystallinity) and heterogeneous fiber formation technologies, it is possible to achieve wide-ranging customization of mechanical properties from flexible gels that simulate soft tissues (whose soft matrix optimizes cell adhesion and force signal transduction by altering the local mechanical environment and binding site density) to rigid scaffolds with osteogenic structures. For instance, fat substitute materials need to have a low modulus (<1MPa) and high ductility, while mandibular angle shaping materials require a modulus of several hundred MPa to maintain structural stability.
Therefore, the core of the design of regenerative medical materials has evolved into "function-oriented mechanical adaptation": that is, based on the anatomical functions of specific areas, the repair cycle and the mechanical microenvironment, the material structure is designed in an inverse manner to achieve "on-demand allocation" and "dynamic evolution" of mechanical support, thereby ensuring safety while achieving the optimal aesthetic and functional reconstruction.

04. Excellent processing performance
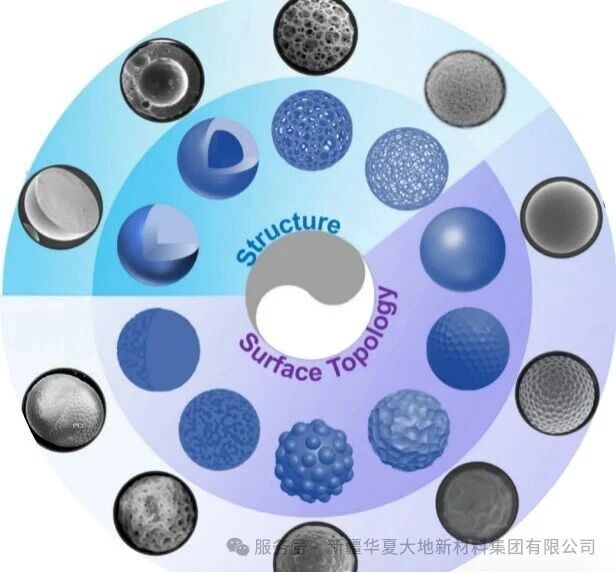
In the fields of regenerative medicine, drug delivery and advanced cosmetic surgery, degradable polyester materials are demonstrating broad design and innovation prospects. This is attributed to their outstanding processing adaptability, which enables them to flexibly integrate with various advanced manufacturing processes, thereby precisely constructing complex structures ranging from nanoscale to centimeter-scale (from microfibers to customized implants). As shown in the figure below, these are microspheres of degradable polyester materials prepared through different processes, each with distinct structures and morphologies.